Amiodarone hydrochloride: Mechanism, Pharmacokinetic Properties and Photosensitivity
Introduction
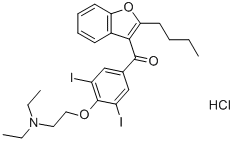
Amiodarone hydrochloride (2-butyl-3-benzofuranyl 4-[2-(diethylyamino)-ethoxy]-3-5-diiodophenyl ketone HCL, molecular weight=681.8; Figure 1) was developed by R. Charlier at Labaz Laboratories in Belgium. It is a di-iodinated benzofuran derivative, containing a diethylated tertiary amine chain. Originally recognized to be an antianginal agent, within 2 years its antiarrhythmic abilities had become appreciated. Early investigations were primarily confined to Europe and South America, where the use ofamiodarone as an antianginal and antiarrhythmic gained widespread acceptance.

The pharmacokinetics of amiodarone and its metabolites are unlike those observed with any of the currently available antiarrhythmics (of any class).This is reflected by early clinical reports using amiodarone in which the number of dosing regimens appear to be only surpassed by the number of investigators. Although amiodarone hydrochloride has been existent since the early 1960s, an understanding of its biological/metabolic fates and kinetics has emerged only within the last decade. This knowledge can be put to sound clinical use to improve our understanding of appropriate loading (acute care) and maintenance therapy.[1]
Mechanism of Action
The Vaughan Williams classification system imperfectly classifies the medication as a class III antiarrhythmic because of its predominant potassium channel blockade that increases the cardiac action potential duration. Amiodarone hydrochloride inhibits potassium efflux through IKr, or rapid delayed rectifier channel, during phase III of the action potential. The medication also has class I antiarrhythmic properties via inhibition of sodium channels during phase 0 of the cardiac action potential. [2, 3]In addition, it results in noncompetitive β-receptor blockade (class II antiarrhythmic activity) and L-type (slow) calcium channel blockade (class IV antiarrhythmic activity). During loading, the acute effects of intravenous amiodarone hydrochloride are predominantly sodium-channel, β-receptor, and calcium channel blockade. The class III effect is seen after completion of the loading dose because of increased levels of the active metabolite, desethylamiodarone.[4]
Pharmacokinetic Properties
Amiodarone hydrochloride has an oral bioavailability of 30–50%, and the rate and extent of amiodarone absorption are increased when taken with food. When taken with a diet rich in fat, the absorption is enhanced by 2.4- to 3.8-fold compared with the fasting state. Therefore, we recommend that amiodarone hydrochloride should be taken with meals. Amiodarone hydrochloride is metabolized in the liver to desethylamiodarone[3]. During loading, it goes through three phases of distribution: (1) central or vascular distribution occurs over approximately 24 h, (2) peripheral or solid organ distribution occurs over the next 7 days, and(3) deep or fat tissue distribution occurs over the subsequent 4 weeks. The full antiarrhythmic effect of amiodarone hydrochloride for VA plateaus after 10 weeks of therapy[3]. The amount of time the medication persists in the body is dependent on the fat content and prior concentration. Amiodarone leaves the body in the reverse order of distribution, which can take several weeks, with a half-life of 50–60 days.
The long half-life of amiodarone hydrochloride means that if it is withheld for a short period of time (up to 1 month) during longterm maintenance therapy, replacement therapy is not necessary. After amiodarone is discontinued, it may continue to have an effect for up to 3 months because of the prolonged half-life. Therefore, it is reasonable to withhold amiodarone if severe toxicity is suspected and confirmatory testing is performed; however, clinicians should be aware that it may take months for amiodarone hydrochloride to clear the body.[4]
Amiodarone photosensitivity
Previous reports from Europe indicate that amiodarone hydrochloride occasionally causes a cutaneous photosensitivity reaction that may be associated with a peculiar blue-gray discoloration of the skin. In addition, corneal microdeposits of yellow-brown granules may occur. Here report observations on a case of amiodarone hydrochloride photosensitivity and corneal deposits developing in a patient shortly after amiodarone therapy was begun. Symptoms included burning and stinging of the skin, with redness and swelling that developed immediately after sun exposure. Phototesting showed that the photoactivating wavelengths were primarily in the long-wave UV-A spectrum between 350 and 380 nm. Prior application of a 10% dioxybenzone sunscreen greatly reduced the phototest reaction. Four weeks after the patient stopped taking amiodarone hydrochloride, the UV-A sensitivity was still present but diminished, and by ten weeks it had disappeared. During this time, the corneal deposits were reduced in number. All ten patients we have treated so far with amiodarone hydrochloride for cardiac arrhythmias have shown a similar photosensitivity, indicating that this is probably a phototoxic reaction.[5]
References
[1]Freedman MD, Somberg JC. Pharmacology and pharmacokinetics of amiodarone. J Clin Pharmacol. 1991;31(11):1061-1069. doi:10.1002/j.1552-4604.1991.tb03673.x
[2]Kodama I, Kamiya K, Toyama J. Cellular electropharmacologyof amiodarone. Cardiovasc Res. 1997;35:13–29.
[3]Mitchell LB, Wyse DG, Gillis AM, Duf HJ. Electropharmacology of amiodarone therapy initiation. Time courses of onsetof electrophysiologic and antiarrhythmic efects. Circulation.1989;80:34-42.
[4]Hamilton D Sr, Nandkeolyar S, Lan H, et al. Amiodarone: A Comprehensive Guide for Clinicians. Am J Cardiovasc Drugs. 2020;20(6):549-558. doi:10.1007/s40256-020-00401-5
[5]Walter JF, Bradner H, Curtis GP. Amiodarone photosensitivity. Arch Dermatol. 1984;120(12):1591-1594.
You may like
See also
Lastest Price from Amiodarone hydrochloride manufacturers
US $0.00/kg2025-09-30
- CAS:
- 19774-82-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $490.00-290.00/kg2025-04-21
- CAS:
- 19774-82-4
- Min. Order:
- 1kg
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month