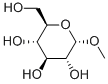
Alpha-D-Methylglucoside: Ionic Current Modulation & Transport Dynamics
Alpha-D-Methylglucoside, as a new type of non-ionic surfactant, has various functions such as strong cleaning power, foaming, lubrication, moisturizing, and emulsification. Different concentrations of methyl α-D-glucopyranoside were used to vary echo decay times in a study that assessed the effects of cryoprotection on the structure and activity of p21ras. Alpha-D-Methylglucoside has also been used in a study to investigate saccharide-mediated protection of chaotropic-induced deactivation of concanavalin A.

Alpha-D-Methylglucoside-induced current in pituitary tumor
Dapagliflozin (DAPA) and canagliflozin (CANA) are selective inhibitors of Na+-dependent glucose co-transporter (SGLT) that can block glucose transport which is highly selective for SGLT2 over SGLT1. Despite their clinical application in type 2 diabetes mellitus patients, there is mounting evidence to show that these agents might produce adverse but yet unexplained effects including hypotension, dizziness, hypoglycemia, decreased renal function and euglycemic diabetic ketoacidosis Therefore, the objective of this study was to explore any possible perturbations of DAPA, CANA or their related compounds on different types of ionic currents in pituitary GH3 cells or in pheochromocytoma PC12 cells. The ionic currents involved in this study include IK(M), erg-mediated K+ current (IK(erg)), hyperpolarization-activated cation current (Ih), voltage-gated Na+ current (INa), and alpha-D-Methylglucoside induced current (IαMG). Findings from this study showed that, in GH3 cells, the presence of DAPA or CANA, in addition to their suppression of SGLT activity, could inhibit IK(M) differentially in a concentration-dependent manner. Moreover, the sugar-induced current (i.e., IαMG) found in GH3 cells can be sensitive to being blocked by both drugs. Therefore, the suppression of sugar-induced current and alpha-D-Methylglucoside induced current might concurrently have important implications on the functional activities of endocrine or neuroendocrine cells.[1]
Under voltage-clamp current recordings, addition of either phlorizin, DAPA or CANA was effective at suppressing IαMG in GH3 cells. Moreover, the Q-V relationship of presteady-state Alpha-D-Methylglucoside induced current in the presence of DAPA shifted along the voltage axis to the rightward and downward direction with no detectable modification in the gating charge of the current. However, cell exposure to tefluthrin, an activator of INa, did not produce any effects on Alpha-D-Methylglucoside induced current, though it can elevate intracellular Na+ concentration. The reduction of IαMG produced by the presence of DAPA or CANA seen in voltage-clamped GH3 cells thus could not result primarily from any changes in intracellular Na+. In our study, under current-clamp conditions, addition of αMG depolarized the cells and raised the AP firing. However, further application of DAPA, CANA or phlorizin, while in the presence of αMG, was noted to increase firing frequency further. The effects by these agents of AP firing seen in GH3 cells can also be attenuated by subsequent addition of flupirtine, an activator of IK(M) (Lu et al., 2019;
Wu et al., 2012). This data is somewhat distinguishable from their resulting effects simply on suppression of Alpha-D-Methylglucoside induced current, since the decreased magnitude of IαMG is expected to hyperpolarize the cells and subsequently diminish AP firing. The most notable explanation for these results, therefore, is that considerable rise in AP firing accompanied by membrane depolarization produced by DAPA, CANA or phlorizin is primarily attributable to the suppression of IK(M) in GH3 cells.
Efflux and the Steady State in alpha-D-Methylglucoside Transport in Escherichia coli
Efflux and the steady state in a group translocation system, the alpha-D-Methylglucoside (αMG) transport system, were investigated. The maximum intracellular level of alpha-D-Methylglucoside is a function of a steady state. There is no inhibition of αMG influx as the intracellular pool of αMG, and α-methylglucoside-6-phosphate (αMGP) rises. This steady state has three components: αMG influx, action of an αMGP phosphatase, and αMG efflux. The phosphatase is the rate-limiting step (half-time = 5.0 min); thus, the true efflux rate (half-time = 2.0 min) cannot be simply measured from the kinetics of αMG loss from the cell. Under our steady-state conditions the percentage of intracellular radioactivity present as αMGP was 71%. Under conditions of zero influx, after an efflux of 12 min the percentage present as αMGP fell to 55%. However, when fluoride was present during the efflux period, the percentage of the sugar as alpha-D-Methylglucoside increased to about 85%. Fluoride greatly inhibits both influx and phosphatase activity (half-time = 50 min). The efflux of αMG from the cell is apparently also fluoride-sensitive but to a lesser extent (half-time = 4.1 min). These data are summarized in a model describing the three components of the steady-state and effect of fluoride.[2]
References
[1] Edmund Cheung So , Sheng-N. W., Ping-Yen Liu. (2020). Effectiveness in the inhibition of dapagliflozin and canagliflozin on M-type K+ current and α-methylglucoside-induced current in pituitary tumor (GH3) and pheochromocytoma PC12 cells. European Journal of Pharmacology, 879, Article 173141.
[2]Winkler HH. Efflux and the steady state in alpha-methylglucoside transport in Escherichia coli. J Bacteriol. 1971 May;106(2):362-8. doi: 10.1128/jb.106.2.362-368.1971. PMID: 4324804; PMCID: PMC285105.
You may like
See also
Lastest Price from alpha-D-Methylglucoside manufacturers

US $0.00/kg2025-08-21
- CAS:
- 97-30-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 2000kgs

US $0.00-0.00/G2025-05-21
- CAS:
- 97-30-3
- Min. Order:
- 10G
- Purity:
- 99%
- Supply Ability:
- 1KG 100KG 1MT