alpha-Arbutin: A Review of its Application as a Skin Lightening Agent
General Description
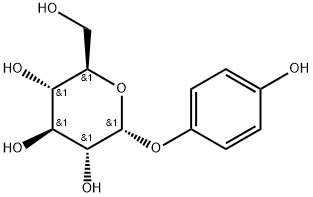
alpha-Arbutin is a hydroquinone glycoside compound, chemical name for 4-hydroxyphenyl-d-glucopyranoside, exists in bear fruit, bilberry and other plants, is a new non-irritating, non-allergic, natural whitening active substances with strong compatibility. alpha-Arbutin has two structural and functional functional groups in its molecular structure: one is a glucose residue; The other is a phenolic hydroxyl group. The physical state of alpha-Arbutin appears as a white to light gray powder, which is more soluble in water and ethanol.

Figure 1. Properties of alpha-Arbutin
Chemical Properties and Safety Profile
alpha-Arbutin, (2R,3S,4S,5R,6R)-2- (hydroxymethyl)-6-(4 hydroxyphenoxy)oxane-3,4,5-triol has a molecular weight of 272.25 g/mol and appears as a white to off-white powder. It has a melting point between 195° – 196°C and a boiling point of 561.6 ± 50.0 °C. The solubility of alpha-Arbutin in water and DMSO is 151 g/L at 20 ± 5 °C and 54 mg/mL (198.34 mM), respectively. It has a log P value of -1.49 and a log S value of -0.84. Although alpha-Arbutin has a strong inhibitory effect on human tyrosinase activity, its high hydrophilicity causes low percutaneous absorption. This limits its permeation into the stratum basale where melanocytes reside. The analysis of alpha-Arbutin can be performed by using HPLC. The stability of alpha-Arbutin is pH-dependent in a buffered aqueous solution, with maximum stability at pH 5.0. The Scientific Committee on Consumer Safety (SCCS) has suggested concentrations of alpha-Arbutin up to 2% in face creams and up to 0.5% in body lotions for the safety of consumers that use alpha-Arbutin based cosmetic products.1-2
Mechanism of Action
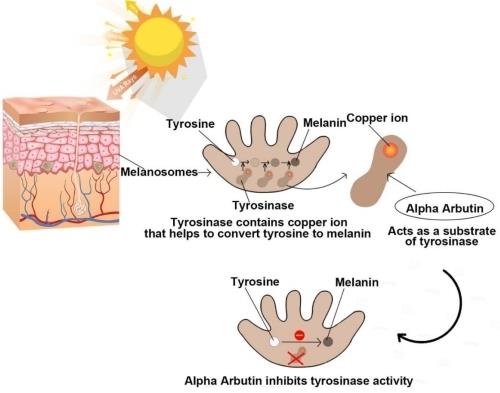
Figure 2. Mechanism of Action of alpha-Arbutin for Skin Lightening
Qin et al., 2014 studied the mechanism of action of alpha-Arbutin by investigating the impact of mushroom tyrosinase on the monophenolase and diphenolase activities. The authors reported that α-arbutin exhibits dual effects on monophenolase and diphenolase activities of mushroom tyrosinase. αarbutin inhibited the reduction of the enzyme activity in the steady-state (suicide inactivation of the active sites of tyrosinase) during the monophenolase reaction. Also, a characteristic lag period was observed during the oxidation of tyrosine during the monophenolase activity. The lag time showed a dose-dependent increase with the increasing concentration of alpha-Arbutin. However, during the diphenolase reaction, αarbutin acted as an activator due to the interaction of α-arbutin with residues located at the entrance to the active site. Herein, no lag period was observed during the oxidation of L-Dopa. Furthermore, it also decreased the effect of suicide inactivation. In light of the above findings, Garcia-Jimenez et al., 2017 carried out experiments to gain in-depth insights into the mechanism of action of alpha-Arbutin. The authors reported that the α-arbutin did not completely inhibit tyrosinase and proposed that α-arbutin acts as an alternative competitive substrate to the enzyme since the enzyme is able to hydroxylate it and does not function as an inhibitor. In another study, Sugimoto et al., 2004 studied the inhibitory effects of alpha-Arbutin on melanin biosynthesis in cultured human melanoma cells and a 3D (three-dimensional) human skin model. The authors reported a concentration-dependent inhibition of melanin synthesis by alpha-Arbutin on human melanoma cells, HMV-II. The inhibitory effect on melanogenesis was achieved at noncytotoxic concentrations of alpha-Arbutin. The authors concluded that direct inhibition of melanosomal tyrosinase activity by alpha-Arbutin hampered the melanogenesis process, rather than suppressing tyrosinase gene expression or cell growth. Another mechanism was proposed by Chakraborty et al., 1998 that the inhibition of tyrosinase activity by alpha-Arbutin might be due to its influence at the post-translational level.3-6
Application
alpha-Arbutin is a chemical that is similar to arbutin, can inhibit the production and deposition of melanin, remove spots and freckles. The results show that arbutin can inhibit the activity of tyrosinase at relatively low concentration, and its inhibitory effect on tyrosinase is better than that of arbutin. alpha-arbutin can be used as a whitening agent in cosmetics.
Lightens dark spots
Alpha-arbutin has been shown to be quite effective in reducing the appearance of dark spots, such as age spots, sunspots, acne scars, melasma, and so on. It evens out the skin tone and gives the appearance of radiance and brightness to the skin.
Diminishes acne scars
Acne scars, which may be either red or brown in colour, are unsightly and can make your skin seem dry and unhealthy. However, alpha-arbutin can help diminish their appearance, giving your skin a more radiant look.
Treats uneven skin tone
The skin will have a more even tone and a more flawless appearance as a result of the effects of alpha-arbutin, which works by inhibiting the synthesis of tyrosinase. In addition, this component addresses a variety of additional skin concerns, including skin pigmentation and a decrease in the skin's elasticity.
Prevents hyperpigmentation
Since alpha-arbutin is able to inhibit the production of melanin, this compound is an excellent choice as an ingredient for products that seek to control hyperpigmentation and discolouration. In addition to this, it is well known to shield the skin from the damaging effects of free radicals without increasing the sensitivity of the skin. A decrease in melanin synthesis leads to a more uniform skin tone, less hyperpigmentation, fewer freckles, and fewer dark patches on the skin.
Moisturizes skin
alpha-Arbutin helps stabilise and maintain the skin, which results in a more even and young appearance.
Side Effects
alpha-Arbutin doesn't interact negatively with any of the skincare ingredients. And it is safe for all skin types as well. Still, there may come a few odd situations wherein it may cause some adverse effects on your skin. Remember, arbutin is a natural yet derivative of hydroquinone. Under alkaline conditions, it can get hydrolyzed to form hydroquinone. However, the skin's surface usually is acidic. So, the probability of such occurrences is relatively low.
Toxicity
In an acute oral toxicity study, the LD50-value for alpha-Arbutin is 9804 mg/kg bw for the mouse and 8715 mg/kg bw for the rat. Dermal LD50 value in rat and mouse was reported to be greater than 928 mg/kg bw, according to an acute dermal toxicity study 7. Extremely high doses may cause ringing in the ears, shortness of breath, convulsions, collapse, vomiting and delirium 6. Nausea and vomiting were seen individuals with sensitive stomachs following oral ingestion of 15 g of dried uva ursi leaves that contain alpha-Arbutin.7
Preparation
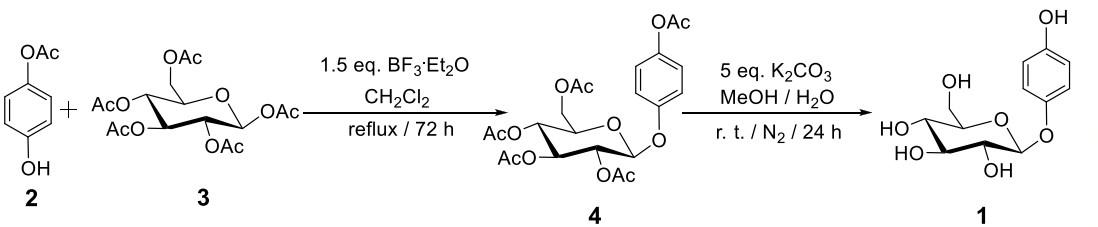
Figure 3. Preparation of alpha-Arbutin
The multi-step alpha-Arbutin synthesis starting from glucose by C. Mannich in 1912 resulted in a poor overall yield, but for the first-time, care was taken to purify the compound. Glucose was first treated with an excess of acetyl bromide in the absence of a solvent, resulting in the per-acylated form with Br at the anomeric center. This glycosyl bromide was then treated with HQ under basic conditions (NaOH), leading to tetra-acetyl arbutin. Finally, hydrolytic deprotection was achieved by treatment with a barium hydroxide solution ("Barytwasser"), followed by acidification using CO2. Subsequent reports appeared in which the Koenigs-Knorr reaction was applied, specifically on the basis of the Helferich variant utilizing phenol-type donors, which proved to be more efficient. A more recent study was published in 2004 that featured a multi-step process including the protection and deprotection of HO-groups in glucose. In yet another study, rather than utilizing the glycosyl bromide, the respective acetate was employed, which offers additional advantages (Figure 3). The glycosylation reaction was performed with the Lewis acid BF3·OEt2 (55–62% yield) followed by complete deprotection (92%).8
References
1. Sugimoto, K., Nishimura, T., & Kuriki, T. (2007). Development of α-Arbutin: Production at Industrial Scale and Application for a SkinLightening Cosmetic Ingredient. Trends in Glycoscience and Glycotechnology, 19(110), 235- 246.
2. Degen, G. H. (2016). Opinion of the Scientific Committee on Consumer safety (SCCS)– Opinion on the safety of the use of α-arbutin in cosmetic products. Regulatory Toxicology and Pharmacology, 74, 75-76.
3. Qin, L., Wu, Y., Liu, Y., Chen, Y., & Zhang, P. (2014). Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase. PLoS One, 9(10), e109398.
4. Garcia-Jimenez, A., Teruel-Puche, J. A., Berna, J., Rodriguez-Lopez, J. N., Tudela, J., & GarciaCanovas, F. (2017). Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS One, 12(5), e0177330.
5. Sugimoto, K., Nishimura, T., Nomura, K., Sugimoto, K., & Kuriki, T. (2004). Inhibitory Effects of α-Arbutin on Melanin Synthesis in Cultured Human Melanoma Cells and a ThreeDimensional Human Skin Model. Biological and Pharmaceutical Bulletin, 27(4), 510-514.
6. Chakraborty, A. K., Funasaka, Y., Komoto, M., & Ichihashi, M. (1998). Effect of arbutin on melanogenic proteins in human melanocytes. Pigment cell research, 11(4), 206-212.
7. Chemical Information Review Document for Arbutin [CAS No. 497-76-7] for Arbutin and Extracts from Arctostaphylos uva-ursi.
8. Zhou, H., Zhao, J., Li, A., & Reetz, M. T. (2019). Chemical and biocatalytic routes to arbutin. Molecules, 24(18), 3303.
You may like
Related articles And Qustion
See also
Lastest Price from alpha-Arbutin manufacturers

US $0.00/kg2025-08-29
- CAS:
- 84380-01-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $0.00-0.00/kg2025-08-21
- CAS:
- 84380-01-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1