Acalabrutinib:Brand name, FDA approval, Indications and Side effects
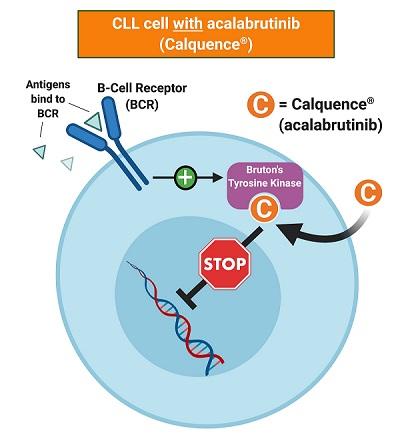
Acalabrutinib belongs to a group of targeted drugs known as cancer growth blockers. It is a type of targeted cancer drug called a bruton tyrosine kinase inhibitor.

Brand name
Calquence, AstraZeneca
FDA approval
On January 16, 2025, the Food and Drug Administration granted traditional approval to acalabrutinib with bendamustine and rituximab for adults with previously untreated mantle cell lymphoma (MCL) who are ineligible for autologous hematopoietic stem cell transplantation (HSCT).
FDA also granted traditional approval to acalabrutinib as a single agent for adults with previously treated MCL. Acalabrutinib receivedExternal Link Disclaimer accelerated approval for this indication in 2017.
Indications
Acalabrutinib is an oral inhibitor of Bruton's tyrosine kinase that is used in the therapy of B cell malignancies including refractory mantle cell lymphoma and chronic lymphocytic leukemia.
Side effects
More common adverse reactions:
Constipation
difficulty in moving
joint pain or swelling
muscle cramps or stiffness
rash
Serious adverse reactions occurred in 69% of patients with acalabrutinib plus BR, and fatal adverse reactions occurred in 12%. Serious adverse reactions reported in ≥2% of patients were pneumonia, COVID-19, pyrexia, second primary malignancy, rash, febrile neutropenia, atrial fibrillation, sepsis, and anemia.
Mechnaism
Tyrosine kinase inhibitors (TKIs) block chemical messengers (enzymes) called tyrosine kinases. Tyrosine kinases help to send growth signals in cells, so blocking them stops the cell from growing and dividing.
Hepatotoxicity
In open label clinical trials of acalabrutinib in patients with CLL and mantle cell lymphoma, serum aminotransferase elevations occurred in 19% to 23% of patients during therapy and rose to above 5 times ULN in 2% to 3%. These elevations were transient and resolved spontaneously but occasionally led to early drug discontinuation. Among the 610 patients treated with acalabrutinib in pre-registration trials, there were no instances of clinically apparent liver injury attributed to its use, but there was a single instance of acute liver failure and death due to reactivation of hepatitis B. Similar cases of reactivation have been reported with ibrutinib, another small molecule inhibitor of Bruton's tyrosine kinase. Experience with acalabrutinib has been limited and the frequency of clinically apparent liver injury and reactivation of hepatitis B are not known. The majority of cases have occurred in patients taking multiple immunosuppressive agents and not just acalabrutinib alone.
Related articles And Qustion
Lastest Price from Acalabrutinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 1420477-60-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00-0.00/Kg/Bag2025-04-21
- CAS:
- 1420477-60-6
- Min. Order:
- 1Kg/Bag
- Purity:
- 0.99
- Supply Ability:
- 20 tons