A small ring derivative herbicide-Cyclopyranil
Brief introduction
Cyclopyranil was announced by Kyoyu Agri as a new herbicide in 2017. It is a close analog of pyraclonil (100) used to control weeds in paddy-field rice. The herbicidal activity of both active ingredients is due to their inhibition of protoporphyrinogen-IXoxidase (PPGO), the last enzyme in the porphyrin pathway common to chlorophyll and heme synthesis.
![1H-Pyrazole-4-carbonitrile, 1-(3-chloro-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridin-2-yl)-5-[(cyclopropylmethyl)amino]- Article illustration](/NewsImg/2024-02-19/6384393474542504884213100.png)
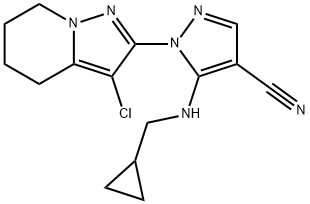
As a Pyrazole-based herbicide, it controls Monochoria vaginalis, Barnyard grass, Crabgrass, and Amaranthus viridis. It also has a good control effect on weeds in dry field crops. This compound was first discovered by Schering (now Bayer), which developed Pyraclonil in 1994.
Synthesis method
The synthesis of cycloguanil starts with the aluminum trichloride-catalyzed acylation of 1,1-dichloroethylene with 5-chlorovaleroyl chloride (101) to afford 1,1,7-trichloro-1-hepten-3-one (102). Its reaction with excess hydrazine hydrate gives the hydrazinopyrazole 103 via a pyrazole ring formation and chloride displacement with hydrazine in one step. The second pyrazole ring is then introduced by transforming 103 with ethoxymethylenemalononitrile[1]. The remaining free position in the tetrahydropyrazolopyridine moiety of the resulting pyrazolylpyrazole derivative 104 is then chlorinated with thionyl chloride to afford the intermediate 105, which delivers cyclopyranil by alkylation of the amino function with chloromethylcyclopropane under primary phase-transfer conditions.
![1H-Pyrazole-4-carbonitrile, 1-(3-chloro-4,5,6,7-tetrahydropyrazolo[1,5-a]pyridin-2-yl)-5-[(cyclopropylmethyl)amino]- Article illustration](/NewsImg/2024-02-19/6384393494648651117821621.png)
References
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.