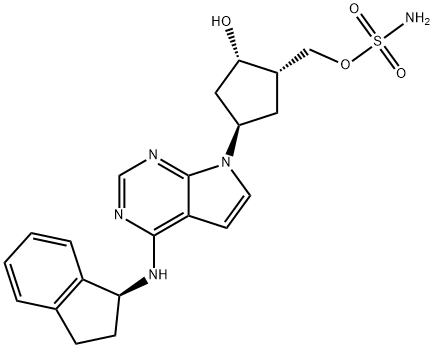
A Neddylation inhibitor: Pevonedistat
Introduction
MLN4924 is a neddylation inhibitor currently under investigation in multiple phases I studies on various malignancies, and its clinical name is Pevonedistat[1]. The NEDD8-activating enzyme is required for the ubiquitination and proteasomal destruction of a large number of proteins. Preclinical research suggested efficacy in treating myeloid neoplasms, prompting its evaluation in human clinical trials. Pevonedistat was subsequently studied in combination with azacitidine in higher-risk MDS, CMML, and AML. Despite early data suggesting more excellent activity with this combination, a recent phase III study did not find any benefit compared to azacitidine alone, underscoring the need for new therapies in MDS and ways to learn from the experiences of randomized trials in this space[2].
The FDA has granted breakthrough therapy designation to pevonedistat for the treatment of patients with higher-risk myelodysplastic syndromes (HR-MDS), according to an announcement from Takeda Pharmaceutical Company Limited.
Mechanism of action
MLN4924 is a specific small molecule inhibitor of NAE. It has been advanced into several phase I clinical trials for certain solid tumours and hematologic malignancies because of its significant anticancer efficacy in preclinical studies. The underlying mechanism of MLN4924 has been thought to be its inhibitory effects on NAE activities by binding to NAE to create a covalent Nedd8-MLN4924 adduct. Consequently, MLN4924 efficiently blocks the neddylation of all Cullins, leading to the accumulation of their substrates, which in turn triggers DNA replication stress, DNA damage response, cell-cycle arrest, apoptosis, autophagy, and senescence, collectively suppressing the growth of cancer cells.
Anticancer agent
Neddylation is a post-translational protein modification associated with cancer development. Neddylation pathway components and CRL1/SCF E3 ligase are potential anticancer biomarkers, to which MLN4924 could serve as a promising drug for cancer therapy. In renal cancer, a cancer type highly resistant to chemotherapy, the efficacy of MLN4924 is unknown but may be of significant interest. The data of Tong et al. showed that MLN4924 markedly inhibited the growth of renal cancer cells by blocking Cullin1 neddylation and subsequent accumulation of their substrates. This led to a DNA damage response, G2-M cell cycle phase arrest and apoptosis. Moreover, they found that MLN4924 blocked the migration of renal cancer cells by upregulating E-cadherin and repressing Vimentin. The study thus provides proof-of-concept evidence for the clinical investigation of this first-in-class anticancer agent in treating renal cancers.
Clinical trial
Pevonedistat is a novel inhibitor of the NEDD8-activating enzyme which, when inhibited, has been seen to impair the viability of AML cells and cause apoptosis. Early-phase clinical trials in pevonedistat in MDS and AML showed safety and efficacy[3]. Further, a phase II study randomized HR-MDS, CMML and low blast count AML to receive pevonedistat plus azacitidine or azacitidine alone. Median overall survival for HR-MDS in the combination group was 23.9 months vs. 19 months with azacitidine alone, with a P value of .334. Event-free survival, however, was significantly longer in HR-MDS with combination group, 20.2 months vs. 14.8 months with azacitidine alone, P value .045. The safety profile was comparable with no increased risk of myelosuppression, and the azacitidine dose was maintained. Because of these findings, Pevonedistat is currently in a phase III randomized clinical trial in high-risk MDS.
References:
[1] SHUAI TONG. MLN4924 (Pevonedistat), a protein neddylation inhibitor, suppresses proliferation and migration of human clear cell renal cell carcinoma.[J]. ACS Applied Electronic Materials, 2017. DOI:10.1038/s41598-017-06098-y.[2] NATALIE M CZUCZMAN. Pevonedistat, a NEDD8-activating enzyme inhibitor, is active in mantle cell lymphoma and enhances rituximab activity in vivo.[J]. Blood, 2016, 127 9 1: 1128-1137. DOI:10.1182/blood-2015-04-640920.
[3] JENNIFER FERRIS. Pevonedistat (MLN4924): mechanism of cell death induction and therapeutic potential in colorectal cancer.[J]. ACS Applied Nano Materials, 2020. DOI:10.1038/s41420-020-00296-w.
You may like
See also
Lastest Price from Pevonedistat manufacturers

US $0.00-0.00/KG2025-04-16
- CAS:
- 905579-51-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000

US $1.00/g2024-10-22
- CAS:
- 905579-51-3
- Min. Order:
- 1g
- Purity:
- 85.0-99.8%
- Supply Ability:
- 20tons