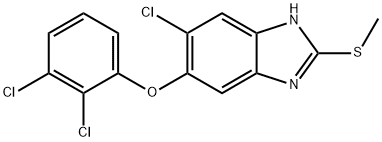
A brief introduction to triclabendazole
Description
Triclabendazole, also known as CGA 89317 or CGP 23030, is an orally bioavailable anthelmintic against infections caused by Fasciola species. Developed by Novartis, triclabendazole received USFDA approval and is the only approved treatment for fascioliasis, a neglected tropical disease impacting 2.4 million patients six years of age and older worldwide.
Mechanism of action
Listed as an Essential Medicine by the World Health Organization (WHO), the drug’s mechanism of action against immature and mature worms of Fasciola hepatica and Fasciola gigantica has not yet been fully elucidated. However, in vitro and in vivo studies in infected animals have suggested that triclabendazole and its two primary metabolites, the sulfoxide and sulfone derivatives, inhibit tubulin polymerization and protein and enzyme synthesis.
Synthesis method
Compared with Ciba Geigy AG, a more recent process disclosed by SeQuent Scientific Limited is more commercially scalable, environmentally friendly, and cost-effective[1]. As shown below, substituting aryl chloride 54 with phenol 55 yielded 87% yield to generate diaryl ether 56. Nitro reduction followed by deacetylation furnished diamine 57, treated with carbon disulfide in methanolic sodium hydroxide to secure thiobenzimidazolone 58 in excellent yield from compound 56. Methylation with dimethyl sulfate (59) and subsequent treatment with base and palladium on carbon in warm toluene-furnished triclabendazole.
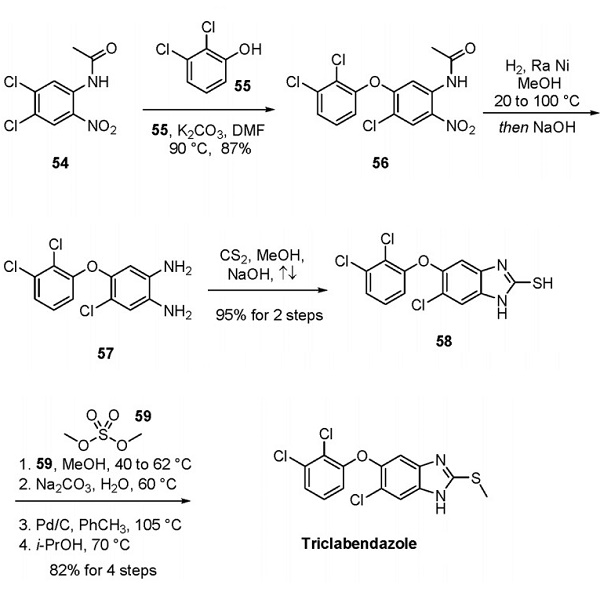
Recrystallization from isopropanol furnished the final product with an 82% yield over the last four operations. Although the role of Pd/C in this final sequence is not explicitly stated in the patent, presumably the Pd/C is used in the third step to avoid any oxidation products before the recrystallization. The published routes by Ciba Geigy AG also furnish triclabendazole via methylation of thiobenzimidazolone 58 using 59, followed by treatment with Pd/C under refluxing conditions.
References
[1] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from Triclabendazole manufacturers

US $5.00-0.50/KG2025-05-09
- CAS:
- 68786-66-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 68786-66-3
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 2ton/month