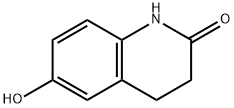
6-Hydroxy-2(1H)-3,4-dihydroquinolinone: Key Intermediate and Efficient Synthetic Strategies
Significance in Medicinal Chemistry
The dihydroquinolinone scaffold, the core structure of 6-Hydroxy-2(1H)-3,4-dihydroquinolinone, is considered a "privileged scaffold" in medicinal chemistry. This term denotes a molecular framework that is able to bind to multiple biological targets, making it a valuable starting point for the development of new drugs. The versatility of the quinoline scaffold allows for the creation of diverse libraries of compounds for drug discovery. Its structure can be readily modified, enabling researchers to fine-tune its properties to target specific biological pathways. This adaptability has led to the development of numerous quinoline-based drugs, with many more currently in clinical trials for various diseases. [1]

Key Intermediates in Drug Synthesis
6-Hydroxy-2(1H)-3,4-dihydroquinolinone has commercial importance as a key intermediate in the preparation of cilostazol. Cilostazol (6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone) is used to treat symptoms of intermittent claudication in patients suffering from symptoms of the disease, which include pain and cramping while walking due to reduced blood flow to the legs. In addition, it serves as a crucial precursor in the synthesis of various pharmacologically active compounds, including antithrombotic agents, anticoagulant kinase inhibitors, anti-inflammatory drugs, anti-ulcer agents, anti-asthmatic drugs, cardiotonic agents, and drugs targeting cerebral circulation, as well as agrochemicals such as herbicides. [2]
Synthesis method
Intramolecular Friedel-Crafts Alkylation of N-(4-methoxyphenyl)-3-chloropropionamide
The present invention provides a process for preparing 6-Hydroxy-2(1H)-3,4-dihydroquinolinone by intramolecular Friedel-Crafts alkylation of N-(4-methoxyphenyl)-3-chloropropionamide in which an equivalent of N-(4-methoxyphenyl)-3-chloropropionamide is contacted with about 3 to about 5 equivalents of a Lewis acid in DMSO or a high boiling amide or amine at an elevated temperature of from about 150° C. to about 220° C. A highly concentrated reaction mixture causes a fast reaction rate yet remains fluid throughout the reaction. The process produces 6-HQ in high yield and a high state of purity such that it may be used in subsequent reactions toward the preparation of cilostazol without intermediate purification. [2]
An Efficient Pd-Catalyzed Synthesis of 6-Hydroxy-2(1H)-3,4-dihydroquinolinone via Intramolecular Cyclization
An alternative method establishes an efficient and industrially applicable Pd-catalyzed strategy for preparing 6-Hydroxy-2(1H)-3,4-dihydroquinolinone. The process begins with the acylation of p-alkoxyaniline (R = Me or Et) with 3-chloropropionyl chloride in the presence of sodium carbonate, typically in organic solvents such as toluene, dichloromethane, or ethyl acetate. This reaction, conducted at approximately 50 °C for several hours, affords the key intermediate N-(4-alkoxyphenyl)-3-chloropropenamide in good yield. The intermediate is subsequently subjected to a palladium chloride–catalyzed intramolecular cyclization and dealkylation in tetrahydrofuran, under moderate pressure (3–5 kg) and elevated temperature (100–110 °C) for 3–4 hours. This step efficiently constructs the quinolinone core while eliminating the alkoxy substituent, thereby generating the target scaffold in a single transformation. Following reaction completion, the crude product is purified by recrystallization from alcohols (ethanol, methanol, or isopropanol) and decolorized with activated carbon to afford a white crystalline solid. The final compound exhibits distinctive XRPD peaks, confirming its structural identity and purity. Compared with conventional multi-step methods, this approach features simplified operations, high overall yield, lower production costs, and scalability, making it particularly suitable for industrial applications. Importantly, the method provides a practical route to a crucial intermediate for cilostazol and other bioactive quinolinone derivatives. [3]
Preparation from 3-Chloropropionyl Chloride
In the existing preparation method of 6-Hydroxy-2(1H)-3,4-dihydroquinolinone, the hydrolysis reaction yield of the alkoxy compound is low, the cyclization reaction is difficult to carry out, and nitrobenzene is used as the solvent. Not obvious, resulting in low synthesis efficiency. The invention belongs to the field of preparation of intermediates in chemical engineering, and particularly relates to a preparation method of 6-Hydroxy-2(1H)-3,4-dihydroquinolinone. The preparationmethod comprises the following steps of using aniline and 3-chloropropionyl chloride as raw materials; performing cyclization, nitrification, reduction and diazotization hydrolysis, so as to synthesize the target product, namely 6-Hydroxy-2(1H)-3,4-dihydroquinolinone. The preparation method has the advantages that the reaction conditions are mild, the cost is reduced, and the yield rate is increased. [4]
References:
[1] Yadav, P., & Shah, K. (2021). Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorganic chemistry, 109, 104639. https://doi.org/10.1016/j.bioorg.2021.104639
[2] Mendelovici, M., Pilarsky, G., Nidam, T., & Dolitzky, B.-Z. (2005). Processes for preparing 6-hydroxy-3,4-dihydroquinolinone, cilostazol and N-(4-methoxyphenyl)-3-chloropropionamide (U.S. Patent No. 6,967,209). U.S. Patent and Trademark Office. https://patents.google.com/patent/US6967209B2/en
[3] Nanjing Yuanshu Pharmaceutical Technology Co., Ltd. (2021). An efficient Pd-catalyzed synthesis of 6-hydroxy-3,4-dihydro-2(1H)-quinolinone via intramolecular cyclization (Chinese Patent No. CN108383781B). China National Intellectual Property Administration. https://patents.google.com/patent/CN108383781B
[4] Preparation method of 6-hydroxy-3,4-dihydro-2(1H)-quinolinone, Patent CN 108383781B (Application No. CN 201810143902.9A, filed July 3, 2018; issued July 27, 2021).
Lastest Price from 6-Hydroxy-2(1H)-3,4-dihydroquinolinone manufacturers
US $2.20-8.80/kg2025-06-13
- CAS:
- 54197-66-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 1000kg

US $9.00/KG2024-10-11
- CAS:
- 54197-66-9
- Min. Order:
- 1KG
- Purity:
- 99.8%
- Supply Ability:
- 100tons