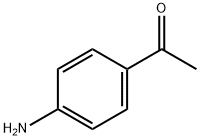
4-Aminoacetophenone: properties and applications in versatile fields
General Description
4-Aminoacetophenone is a white to pale yellow crystalline solid with a melting point of 96-100°C. It has limited solubility in water but dissolves well in organic solvents like ethanol and acetone. 4-aminoacetophenone is a versatile compound with various applications. It can be used in the synthesis of anti-inflammatory pyrimidines, which exhibit potent effects. It also shows promise in regulating the activity of the enzyme tyrosinase, potentially surpassing kojic acid as an inhibitor. Additionally, it plays a role in food safety by enabling rapid and sensitive nitrite detection in pickled vegetables. Overall, 4-aminoacetophenone offers opportunities for pharmaceutical and analytical applications.

Figure 1. 4-Aminoacetophenone
Properties
4-aminoacetophenone is a white to pale yellow crystalline solid with a characteristic odor. It has a melting point ranging from 96 to 100 degrees Celsius and exhibits limited solubility in water but good solubility in organic solvents like ethanol, acetone, and chloroform. The compound has a boiling point of approximately 270 degrees Celsius and a density of 1.161 g/cm³. With a molar mass of around 135.17 grams per mole, its chemical structure consists of a benzene ring substituted with an amino group at the para position and an acetyl group attached to the amino group. Due to its aromatic ketone nature, 4-aminoacetophenone displays typical reactivity and can undergo reactions such as nucleophilic substitution, oxidation, and condensation with suitable reactants. 1
Applications in versatile fields
Synthesis of anti-inflammatory agent
Chalcone derivatives were synthesized by reacting 4-aminoacetophenone with different substituted aromatic and heteroaromatic aldehydes using Claisen-Schmidt condensation. These chalcones were then subjected to a reaction with guanidine hydrochloride under basic alcoholic conditions, leading to the formation of 2,4,6-trisubstituted pyrimidines in excellent yields. The newly synthesized pyrimidines were characterized using various techniques such as IR spectroscopy, ¹H- and ¹³C-NMR spectroscopy, Electron Ionization (EI)-mass spectrometry, and elemental analyses. In vivo screening tests were conducted to evaluate the anti-inflammatory and analgesic activities of the compounds. Among the tested pyrimidines, 2-amino-4-(4-aminophenyl)-6-(2,4-dichlorophenyl)pyrimidine (5b) and 2-amino-4-(4-aminophenyl)-6-(3-bromophenyl) pyrimidine (5d) exhibited the most potent anti-inflammatory and analgesic activities compared to the reference standard ibuprofen. 2
Tyrosinase activators and inhibitors
In a study, the application of 4-aminoacetophenone and its derivatives were investigated as tyrosinase activators and inhibitors. The researchers synthesized 4-aminoacetophenone and their structure-based 4-aminophenylethylidenethiosemicarbazide derivatives. Interestingly, all the thiosemicarbazone compounds exhibited stronger tyrosinase inhibitory activities than kojic acid, a commonly used inhibitor. Particularly, compound 7k demonstrated the highest potency as a tyrosinase inhibitor, with an IC50 value of 0.291 μM. Furthermore, the researchers explored the inhibition mechanism and kinetics. Compound 7k was identified as a reversible and non-competitive inhibitor, while compound 8h exhibited reversible and competitive-uncompetitive mixed-II type inhibition. These findings contribute to the understanding of the potential applications of 4-aminoacetophenone and its derivatives in regulating tyrosinase activity. 3
The detection of nitrite in food
The rapid and colorimetric method developed for nitrite detection in food using 4-aminoacetophenone and propylene glycol alginate (PGA) gel interface as a reaction medium offers significant advantages. Nitrite reacts with 4-aminoacetophenone, forming a diazo compound that further reacts with N-(1-Naphthyl)ethylenediamine (NED), resulting in a purplish-red compound. The concentration of nitrite correlates linearly with the grayscale value of the PGA gel interface within the range of 0.3-9 μg mL-1. The method exhibits high sensitivity with a detection limit of 0.3 μg mL-1. When applied to pickled vegetables, it achieved satisfactory recovery rates ranging from 80.9% to 119.02%, with a relative standard deviation between 0.11% and 6.73%. Notably, the entire detection process takes only 5 minutes, showcasing its efficiency. Overall, this colorimetric approach offers simplicity, rapidity, and high sensitivity, making it ideal for real-time and on-site nitrite detection in pickled vegetables. Its implementation contributes significantly to ensuring food safety and quality. 4
Reference
1. Pubchem. Compound Summary: 4-Aminoacetophenone. National Library of Medicine, 2005, CID:7468.
2. Yejella RP, Atla SR. A study of anti-inflammatory and analgesic activity of new 2,4,6-trisubstituted pyrimidines. Chem Pharm Bull (Tokyo), 2011, 59(9):1079-1082.
3. You A, Zhou J, Song S, Zhu G, Song H, Yi W. Structure-based modification of 3-/4-aminoacetophenones giving a profound change of activity on tyrosinase: from potent activators to highly efficient inhibitors. Eur J Med Chem, 2015, 93:255-262.
4. Zhang J, Yang J, Chen J, Zhu Y, Hu K, Ma Q, Zuo Y. A novel propylene glycol alginate gel based colorimetric tube for rapid detection of nitrite in pickled vegetables. Food Chem, 2022, 373(Pt B):131678.
Related articles And Qustion
See also
Lastest Price from 4-Aminoacetophenone manufacturers
US $0.00/KG2025-05-07
- CAS:
- 99-92-3
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $10.00/ASSAYS2025-05-04
- CAS:
- 99-92-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton