3-Isocyanatopropyltriethoxysilane: Oligomers, Mesoporous Organosilicas and Hg2? Preconcentration
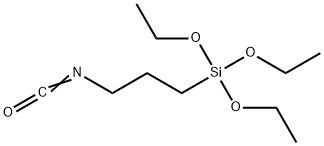
3-Isocyanatopropyltriethoxysilane is an isocyanate functional silane used for the functionalization of compounds with active hydrogen atoms. It has various applications, including surface treatment of organic materials and inorganic metals, particularly in glass fiber reinforced composites and as a coupling agent in coatings and adhesives. It is known for enhancing adhesion and improving the mechanical properties of materials. 3-Isocyanatopropyltriethoxysilane is an organosilicon compound featuring both isocyanate and triethoxysilane functional groups. This dual functionality enables it to act as a coupling agent, enhancing adhesion between organic polymers and inorganic substrates. It is widely utilized in industries such as adhesives, sealants, coatings, and surface treatments.

Formation and Depolymerization of Oligomers
Isocyanates as important organic synthetic intermediates were main raw materials for the synthesis of polyurethane, which could be widely used in polyurethane oligomer materials (such as pesticide, paint, dyes etc.). Among them, 3-isocyanatopropyltriethoxysilane (IPTS) was a new type of organic silane coupling agent, which contains two different kinds of active group ie. isocyanate group (NCO) and ethoxy (OC2H5). IPTS was widely used in polyethylene crosslinking, surface treatment of glass fiber reinforced plastics (such as unsaturated resins, polyethylene, polypropylene resins), surface moisture-proof treatment of electronic components, surface treatment of inorganic silicon filler, special coatings and adhesives. Besides, 3-Isocyanatopropyltriethoxysilane plays a significant coupling role in improving the product mechanical, electrical, water resistant, anti-aging properties. In order to improve the rate of pyrolysis, this process usually conducted at high temperatures. Probably because the higher cracking temperature, the polymerization reactions between N-substituted carbamates and isocyanates were easy to occur. The formation of polyureas, isocyanurate trimer and polycarbodiimide will significantly reduce the yield of isocyanates, which vastly hinder the industrial application of thermal cracking process. In the process of preparing IPTS by thermal cracking of CPTS, it was found that the thermal stability of IPTS was lower than that of other common diisocyanate.[1]
From the preliminary results, it can be seen that the polymerization rate increased with the increase of temperature from 150 °C to 190 °C (5 % h−1 to 19 % h−1). At the same temperature and time (150 °C, 6 h), the introduction of the SnO(C4H9)2 catalyst could also accelerate the rate of polymerization. The formation of oligomers was influenced by time, temperature and catalyst, but temperature was probably the biggest factor. Besides, the corresponding yield of IPTS was decreased with the rising cracking temperature, since most of the CPTS were rectified with the IPTS fast at high temperature. The catalyst can not only increase the yield of 3-Isocyanatopropyltriethoxysilane but also the yield of oligomers, proper cracking temperature and catalyst must be selected to obtain high yield of 3-Isocyanatopropyltriethoxysilane. The possible polymerization reactions in the process of CPTS thermal cracking and the structure of the corresponding oligomers were investigated. The polyureas mainly comes from the polymerization reaction between isocyanates and carbamates. Fortunately, these oligomers were successfully degraded using urea as carbonyl and catalyst. Recycling of oligomerized substrates will have important significance for achieving the non-phosgene synthesis of isocyanates.
Periodic Mesoporous Organosilicas as Efficient Nanoreactors
To improve the atomic efficiency and decrease the economic cost or undesired intermediate separation processes, the development of a new kind of catalyst is required; one that can connect the two-step reactions in tandem and can be recovered easily from the system for further recycling. In this work, coupling regents of hydroquinone (HQ), bisphenol A (BPA), and 1,1′-bi-2-naphthol (BN) were nucleophilically added to 3-isocyanatopropyltriethoxysilane, and three types of organosilica precursors, denoted as HQP, BPAP, and BNP, respectively, were prepared. Reagents of 3-isocyanatopropyltriethoxysilane (ICPTES), hydroquinone, bisphenol A, 1,1′-bi-2-naphthol, tetraethyl orthosilicate (TEOS), methyl orthosilicate (TMOS), all species of aldehyde, malononitrile, and diethylbromomalonate were purchased from Macklin Industrial Corporation. Pluronic P123 (P123, EO20PO70EO20) was purchased from Aldrich. Anhydrous THF and toluene were obtained by treatment with activated 4 Å molecular sieves for at least one week and were used without further purification.[2]
Three different functionalized organosilica precursors were synthesized and then co-condensed and hydrolyzed with TEOS to prepare periodic mesoporous organosilicas by a hydrothermal method. The material of MC-HQ showed the highest surface area with an ordered two-dimensional hexagonal structure, and the materials of MC-BPA and MC-BN respectively showed disordered and cellular foam structures with lower surface area.
Highly selective preconcentration of Hg2+ in water samples
Inspired by the T–Hg2+–T interaction, we proposed to synthesize a new functional monomer, 3-isocyanatopropyltriethoxysilane (IPTS) bearing T bases (T-IPTS), with T group as recognition element for the imprinting of Hg2+. Then a novel Hg-IIPs based on T–Hg2+–T interactions, Hg-IIPs-T (that is IIPs-T, for simplicity), were prepared by sol–gel process. To the best of our knowledge, this work is the first demonstration of T–Hg2+–T application in IIPs. To further confirm the feasibility of the IIPs-T for the specific uptake of Hg2+, they were used as SPE sorbent for preconcentration of Hg2+ from water samples. Satisfactory recoveries were obtained. Compared to the IIPs based on traditional functional monomer of 4-VP, i.e. IIPs-VP, the novel IIPs-T showed significantly higher selectivity and binding capacity. The excellent selectivity of Hg-IIPs-T can be expected by the specific identification between T and Hg2+. A novel functional monomer T-IPTS, 3-isocyanatopropyltriethoxysilane (IPTS) bearing thymine (T) bases, was synthesized for imprinting Hg2+. Then a novel Hg2+ ionic imprinted polymers (IIPs) based on thymine–Hg2+–thymine (T–Hg2+–T) interactions, i.e. Hg-IIPs-T, were prepared by sol–gel process for the first time in this work. Accordingly, Hg-IIPs-T were used as solid-phase extraction (SPE) sorbents for preconcentration of trace Hg2+ in water samples, and satisfactory recoveries ranging from 95.2 to 116.3% were obtained.[3]
References
[1]Wang P, Zhou D, Fei Y, Long Y, Deng Y. Formation and Depolymerization of Oligomers during Thermal Cracking of Silicon-Containing Carbamates. Chempluschem. 2019 Aug;84(8):1039-1045. doi: 10.1002/cplu.201900245. Epub 2019 Jun 24. PMID: 31943955.
[2]Huo H, Jiang Y, Zhao T, Wang J, Li D, Xu X, Lin K. Periodic Mesoporous Organosilicas as Efficient Nanoreactors in Cascade Reactions Preparing Cyclopropanic Derivatives. Chem Asian J. 2019 May 2;14(9):1496-1505. doi: 10.1002/asia.201900043. Epub 2019 Mar 21. PMID: 30803135.
[3]Xu S, Chen L, Li J, Guan Y, Lu H. Novel Hg2+-imprinted polymers based on thymine-Hg2+-thymine interaction for highly selective preconcentration of Hg2+ in water samples. J Hazard Mater. 2012 Oct 30;237-238:347-54. doi: 10.1016/j.jhazmat.2012.08.058. Epub 2012 Sep 1. PMID: 22981287.
Lastest Price from 3-Isocyanatopropyltriethoxysilane manufacturers

US $0.00-0.00/kg2025-06-13
- CAS:
- 24801-88-5
- Min. Order:
- 0.0001kg
- Purity:
- 99.99%
- Supply Ability:
- 20000000T

US $1.00/KG2025-04-21
- CAS:
- 24801-88-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt