3-Chloroaniline: Applications in the Chemical Industry, Production Methods and Toxic Properties
General Description
3-Chloroaniline is a vital intermediate in the chemical industry, with applications in the production of herbicides, pharmaceuticals, dyes, pigments, insecticides, and agricultural chemicals. It can be synthesized through various methods, including hydrogenation processes, with high yields. However, 3-Chloroaniline exhibits toxicity, with inhalation, skin contact, and ingestion leading to adverse effects such as methemoglobinemia and skin sensitization. Proper handling, safety precautions, and immediate medical attention are essential when working with this compound to ensure the well-being of individuals and minimize risks. Overall, 3-Chloroaniline plays a crucial role in enhancing agricultural productivity, adding vibrancy to products, and improving human health.

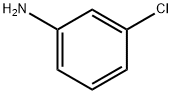
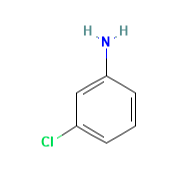
Figure 1. 3-Chloroaniline
Applications in the chemical industry
3-Chloroaniline, a chemical compound, finds diverse applications in the chemical industry. It serves as a crucial intermediate for the production of various products such as herbicides, pharmaceuticals, azo dyes, and pigments. Specifically, it is utilized in the synthesis of herbicides like chlorpropham and m-chlorophenyl isocyanurate, contributing to agricultural productivity through weed control. Furthermore, 3-Chloroaniline plays a significant role in the pharmaceutical industry, serving as an intermediate for the synthesis of pharmaceutical compounds. Its application extends to the production of azo dyes and pigments, adding vibrancy and color to a wide range of products. Additionally, it is used in the manufacturing of insecticides and other agricultural chemicals, further highlighting its importance in enhancing crop yield and protecting crops from pests. Overall, 3-Chloroaniline serves as a versatile building block in the production of various essential products, contributing significantly to the chemical industry. 1
Production methods
3-Chloroaniline, a chemical compound, can be produced using various methods. One commonly used method involves the low-pressure hydrogenation of 3-chloronitrobenzene in the liquid phase. This process utilizes noble metal and/or noble metal sulfide catalysts. To prevent dehalogenation, metal oxides are added to the reaction mixture. The yield of this method is approximately 98%, making it an efficient process. Another mentioned method for producing 3-Chloroaniline is the catalytic hydrogenation of chloronitrobenzene. This method likely utilizes a suitable catalyst to facilitate the reduction reaction. The specific catalyst used may vary depending on the reaction conditions and desired outcome. Additionally, 3-Chloroaniline can be synthesized by reducing chloronitrobenzene with sodium hydrosulfide (NaHS). This method provides an alternative approach to produce 3-Chloroaniline. The choice of production method for 3-Chloroaniline depends on factors such as the availability of reagents and desired reaction conditions. Each of these methods offers a viable means of synthesizing 3-Chloroaniline, allowing for flexibility in production processes. 2
Toxic properties
3-Chloroaniline exhibits potentially toxic properties, and its exposure through various routes can lead to adverse effects. Inhalation of the substance may cause symptoms such as blue lips, fingernails, and skin, dizziness, headache, shortness of breath, nausea, vomiting, convulsions, weakness, confusion, and unconsciousness. Skin contact with 3-Chloroaniline can result in redness, burning sensation, and potential absorption into the body. Eye contact may cause redness and pain. Ingestion of the substance can lead to abdominal pain. One significant toxic effect associated with 3-Chloroaniline is methemoglobinemia, which refers to the increased presence of methemoglobin in the blood. This condition can impair the ability of red blood cells to carry oxygen efficiently, leading to oxygen deprivation in tissues. Methemoglobinemia is considered a primary toxic effect of 3-Chloroaniline. Furthermore, 3-Chloroaniline has the potential to act as a skin sensitizer. This means that it can induce allergic reactions in the skin upon repeated or prolonged exposure. It is crucial to handle 3-Chloroaniline with care and take appropriate safety precautions to minimize exposure. Personal protective equipment, such as gloves and respiratory protection, should be used when working with this chemical compound. Adequate ventilation and containment measures should also be implemented to prevent inhalation or skin contact. In case of accidental exposure or ingestion, immediate medical attention is advised. Overall, the toxicological information highlights the importance of understanding and managing the potential risks associated with 3-Chloroaniline to ensure the safety of individuals working with or around this substance. 3
Reference
1. PubChem Annotation Record for 3-CHLOROANILINE. National Center for Biotechnology Information. 2024, Source: Hazardous Substances Data Bank.
2. 3-Chloroaniline. National Center for Biotechnology Information. 2024. PubChem Compound Summary for CID 7932.
3. 3-Chloroaniline. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases. CAS Number: 108-42-9.
Related articles And Qustion
See also
Lastest Price from 3-Chloroaniline manufacturers

US $1.00/KG2025-04-21
- CAS:
- 108-42-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00/KG2023-10-08
- CAS:
- 108-42-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T