3-Aminopyridine vs 2-Aminopyridine vs 4-Aminopyridine: Difference between the three
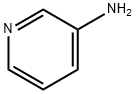
3-Aminopyridine
3-Aminopyridine is an important organic intermediate compound used as a plant growth regulator and also in the manufacture of pharmaceuticals, dyes, agrochemicals and oligomers/polymers. Studies have shown that 3-Aminopyridine has no anti-staphylococcal activity. However, after polymerisation, 3-aminopyridine has strong anti-staphylococcal activity. The oligomers/polymers synthesised from 3-aminopyridine showed varying degrees of antistaphylococcal activity, with the relatively shorter oligomers showing the highest activity. It is therefore concluded that polymerisation of 3-aminopyridine is necessary for the antistaphylococcal activity and the strength of this activity depends on the molecular weight of the synthesised molecules.
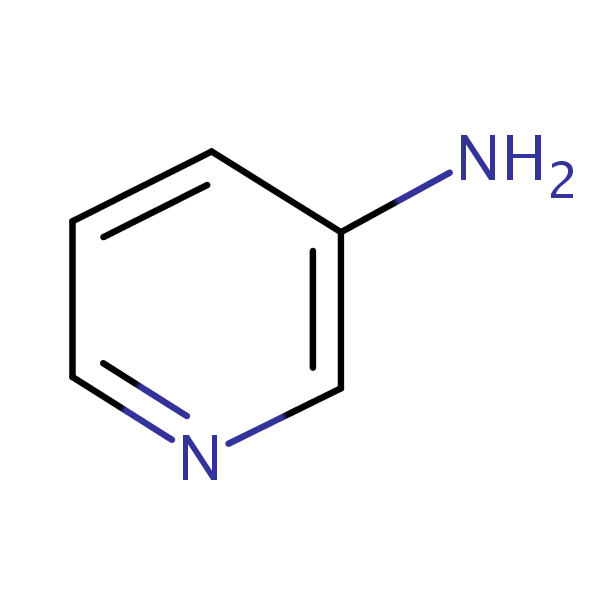
Synthesis of 3-Aminopyridine
3-Aminopyridine is prepared using nicotinamide as a raw material by performing a Hoffman degradation reaction in sodium hypochlorite solution and 10% sodium hydroxide solution. The preparation method specifically includes the following steps:
s1: firstly, 580g of sodium hypochlorite solution with the effective chlorine content of 7.5 percent is subjected to cooling and stirring treatment;
s2: when the temperature of the sodium hypochlorite solution is lower than 20 ℃, adding 75g of solid nicotinamide into the sodium hypochlorite solution, simultaneously keeping the temperature of the reaction solution at 5-10 ℃ so as to carry out low-temperature reaction for 30-60 min, and continuously stirring until the reaction solution is clear;
s3: testing the pH value of the reaction solution, detecting whether the raw materials are completely converted by HPLC, adding 72g of 10% sodium hydroxide solution, adjusting the reaction solution to be strong alkaline, transferring the reaction solution to a water bath, slowly heating to control the temperature to be 70-75 ℃, and keeping the temperature for 1-1.5 h;
s4: sampling and detecting by HPLC (high performance liquid chromatography) at regular time during the reaction period of the step S3 until the content of the product is more than or equal to 98 percent and the reaction is finished;
s5: performing rotary evaporation, concentration and suction filtration on the reaction solution after the reaction in the step S4, and extracting by using an organic solvent to obtain a crude product of the 3-aminopyridine;
s6: and recrystallizing and refining the crude product of the 3-aminopyridine to obtain a light yellow or white solid, and drying in vacuum to obtain the product of the 3-aminopyridine.
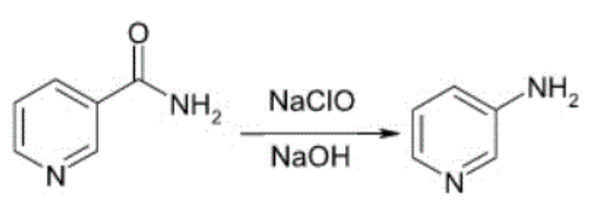
2-Aminopyridine
2-Aminopyridine is a simple, low molecular weight and perfectly functionalized organic compound for the synthesis of a wide range of biomolecules. It is used in the pharmaceutical field as an important component of the pharmacophore against various biological targets and can be used in the preparation of drugs such as piroxicam, sulfapyridine, tenoxicam and triphenamine.
The oxidation of 3-aminopyridine to 3,3′-azoxypyridine by peroxomonophosphoric acid (PMPA) is a total second order reaction: first order each in peroxomonophosphoric acid and 3-aminopyridine at constant acidity. The observed pH-rate profile has been rationalized invoking various PMPA species, protonated and unprotonated forms of 3-aminopyridine as the reactive species and their reactivities have been estimated. Interestingly, 2-aminopyridine is not oxidized in the pH-range where the oxidation of 3-aminopyridine is facile.
The decay dynamics of 2-aminopyridine and 3-aminopyridine excited to the S1 state is investigated using femtosecond time-resolved photoelectron imaging. The lifetime of the S1 state for both molecules shows a rapid decrease with the increase of the vibrational energy. It is shown that, besides intersystem crossing to the lower-lying triplet state of T1, the decay to the ground state (S0) via internal conversion through a conical intersection plays an increasingly important role and becomes dominant for vibrational states well above the S1 state origin. The comparison between 2-aminopyridine and 3-aminopyridine suggests that the intramolecular hydrogen bonding between a hydrogen atom of the NH2 group and the heterocyclic nitrogen atom in 2-aminopyridine effectively hinders the ring deformation at lower vibrational states which is required for the wavepacket to reach the S1/S0 conical intersection, and therefore slows down the S1 to S0 internal conversion.
4-Aminopyridine
4-aminopyridine is a ligand and an ion channel modulator used as an acetylcholine release enhancing agent.
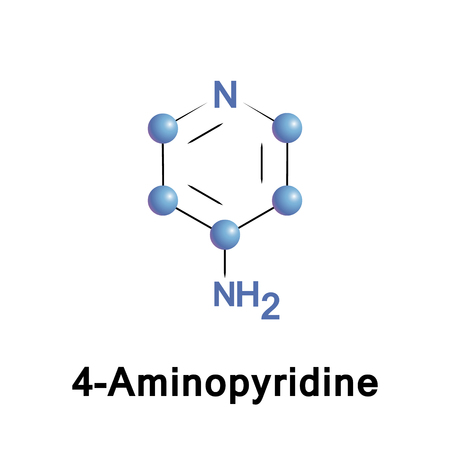
4-Aminopyridine (4-AP) can be used as a:
Catalyst in the regioselective acylation of N-tosylhydrazide.
Starting material in the synthesis of 4-aminopyridine derivatives for neurological disorder studies.
Precursor for the synthesis of enantiomerically pure 4-(pyrrolidino)pyridine (PPY) derivatives by cyclocondensation reaction.
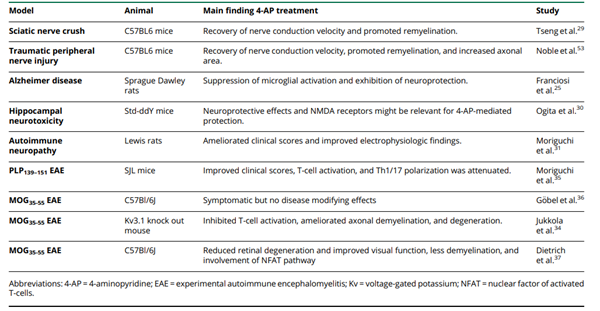
As an antagonist of voltage-gated potassium (Kv) channels, 4-aminopyridine (4-AP) is used as symptomatic therapy in several neurologic disorders. The improvement of visual function and motor skills and relieve of fatigue in patients with MS have been attributed to 4-AP. Its prolonged release formulation (fampridine) has been approved for the symptomatic treatment of walking disability in MS. The beneficial effects were explained by the blockade of axonal Kv channels, thereby enhancing conduction along demyelinated axons. However, an increasing body of evidence suggests that 4-AP may have additional properties beyond the symptomatic mode of action. Below is a summary of the 4-AP preclinical study and key findings:
References:
[1] C. AKGUL M. Y. Molecular weight dependent antistaphylococcal activities of oligomers/polymers synthesized from 3-aminopyridine[J]. Journal of The Serbian Chemical Society, 2010. DOI:10.2298/JSC100201104A.
[2] RAO R N, CHANDA K. 2-Aminopyridine – an unsung hero in drug discovery[J]. Chemical Communications, 2021. DOI:10.1039/D1CC04602K.
[3] WAZZAN N. DFT and MP2 Study of Geometry, IR and UV-Visible Spectroscopy and First Hyperpolarizability of 2-Aminopyridine, 3-Aminopyridine and 4-Aminopyridine in Gas Phase and in Solvents[J]. Asian Journal of Chemistry, 2015. DOI:10.14233/ajchem.2015.19304.
[4] S. N. MAHAPATRO G. P P A Panda. Peroxomonophosphoric Acid Oxidations. VI. Kinetics and Mechanism of Oxidation of 3-Aminopyridine[J]. Bulletin of the Chemical Society of Japan, 1981. DOI:10.1246/BCSJ.54.2507.
[5] MICHAEL DIETRICH Philipp A Hans Peter Hartung. Neuroprotective Properties of 4-Aminopyridine.[J]. Neurology® Neuroimmunology & Neuroinflammation, 2021. DOI:10.1212/NXI.0000000000000976.
[6] FENG B, YANG D, MIN Y, et al. Excitation wavelength dependent S1-state decay dynamics of 2-aminopyridine and 3-aminopyridine†[J]. Physical Chemistry Chemical Physics, 2023. DOI:10.1039/D3CP01487H.
Lastest Price from 3-Aminopyridine manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 462-08-8
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-09
- CAS:
- 462-08-8
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton