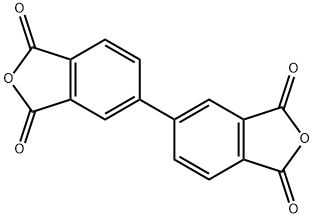
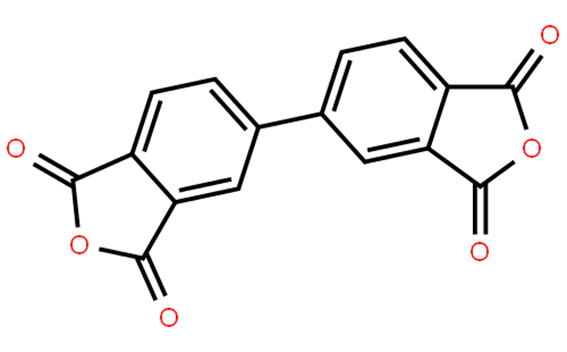
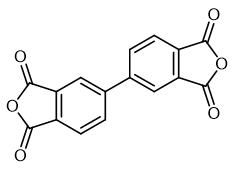
3,3',4,4'-Biphenyltetracarboxylic Dianhydride: Structure-Property Relationship
3,3',4,4'-Biphenyltetracarboxylic dianhydride (s-BPDA) is a rigid symmetric polyamide with a diamine having a long distance between amine groups and reactive endgaps. 3,3',4,4'-Biphenyltetracarboxylic dianhydride can be used in the synthesis of high molecular weight aromatic polyamide fibers. It can also be used in the formation of hybrid nanocomposite films.

Phenylethynyl-Terminated Imide Oligomer-Based Thermoset Resins
Thermosetting polyimides (PIs) based on phenylethynyl-terminated imide (PETI) oligomers are among the most critical high-performance resin matrices for high-temperature aerospace and aircraft applications. Upon curing, PI resins form highly rigid network structures through the additional reaction of phenylethynyl groups, imparting exceptional thermo-oxidative stability, mechanical strength, and resistance to heat and radiation. The PETI-5, derived from 3,3',4,4'-Biphenyltetracarboxylic dianhydride (s-BPDA) and developed at NASA Langley Research Center (LaRC), with a targeted molecular weight of 5000 g mol−1, demonstrates an excellent combination of high fracture toughness, heat resistance, and processability. It exhibits a minimum melt viscosity of 1000 Pa∙s at 371 °C and a cured glass transition temperature (Tg) of 270 °C. Studies by Scola et al. revealed that substituting 3,3',4,4'-Biphenyltetracarboxylic dianhydride units with 4,4′-(hexafluoroisopropylidene)-diphthalic anhydride (6FDA) units significantly reduces the melt viscosity with minimal impact on Tg . The ongoing development of PETI resins with high Tg and low melt viscosities remains a critical focus area. These resins are preferred for their ability to combine desirable properties, including exceptional fracture toughness and resistance to microcracking. This review summarizes recent advances in PETI resins, emphasizing molecular design and the relationships between structure–processing–properties.[1]
Generally, using isomeric monomers results in a less ordered and more flexible three-dimensional structure, enabling the fabrication of processable polyimides without significant loss of thermal stability. For instance, a PETI based on 2,3,3′,4′-biphenyltetracarboxylic dianhydride (a-BPDA) exhibits a higher Tg than one based on 3,3',4,4'-Biphenyltetracarboxylic dianhydride. This difference is attributed to the asymmetric structure of a-BPDA, which suppresses internal rotation around the biphenyl linkage compared to 3,3',4,4'-Biphenyltetracarboxylic dianhydride. The a-BPDA structure requires a larger sweep volume for crankshaft motion, necessitating more energy (i.e., higher temperature), thereby resulting in a higher Tg. Conversely, isomer-based PETIs such as a-BPDA exhibit enhanced processability. For example, TriA-PI, a PETI based on a-BPDA, exhibited improved solubility and reduced viscosity compared to a PETI based on 3,3',4,4'-Biphenyltetracarboxylic dianhydride due to the asymmetric and non-planar structure of a-BPDA. The characteristic charge transfer complex (CTC) in imides is formed through π-electron conjugation in polyimides, where the nitrogen atom acts as the electron donor and the carboxyl group of the dianhydride functions as the electron acceptor. This review has highlighted significant advancements in the development of ultra-high-temperature PETI derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride, emphasizing the complex interactions between their molecular structures, processing conditions, and resulting material properties.
Gas permeability and permselectivity in polyimides based on 3,3',4,4'-biphenyltetracarboxylic dianhydride
Permeation rates of hydrogen, carbon monoxide, carbon dioxide and methane were measured for amorphous films of a series of polyimides prepared from 3,3',4,4'-Biphenyltetracarboxylic dianhydride (BPDA) and each of the following diamines: 4,4′-oxydianiline (ODA), 4,4′-methylenedianiline (MDA), 4,4′-diaminodiphenyl sulfone (DDS), and dimethyl-3,7-diaminodibenzothiophene 5,5′-dioxide (DDBT). The permeability coefficients (P) at 10 atm and 50°C were in the order BPDA-DDBT > BPDA-DDS ≥ BPDA-MDA > BPDA-ODA. On going from BPDA-ODA to BPDA-DDBT, P increased by 6.0- and 9.4-fold for hydrogen and carbon dioxide, respectively. This was due to 1.4- and 3.2-fold increases in the diffusion coefficients accompanied by appreciable decreases in activation energies, and also to a 3.7-fold increase in the solubility coefficients. These results suggest that both high rigidity and bulkiness of a polymer backbone may prevent efficient chain packing and result in a more open structure for gas transport. 3,3',4,4'-Biphenyltetracarboxylic dianhydride- dimethyl-3,7-diaminodibenzothiophene 5,5′-dioxide displayed high permselectivities for hydrogen/methane and carbon dioxide/methane in addition to high permeability coefficients, and has a high potential for use as a membrane material for gas separation.[2]
References
[1]Kim M, Kim K, Lee JH, Jeon E, Song J, Choi J, Yeo H, Nam KH. Phenylethynyl-Terminated Imide Oligomer-Based Thermoset Resins. Polymers (Basel). 2024 Oct 21;16(20):2947. doi: 10.3390/polym16202947. PMID: 39458775; PMCID: PMC11511106.
[2] K. Tanaka. (1989). Gas permeability and permselectivity in polyimides based on 3,3’,4,4’-biphenyltetracarboxylic dianhydride. Journal of Membrane Science, 47 1, Pages 203-215.
You may like
Related articles And Qustion
Lastest Price from 3,3',4,4'-Biphenyltetracarboxylic dianhydride manufacturers
US $0.00-0.00/Kg2025-10-24
- CAS:
- 2420-87-3
- Min. Order:
- 1Kg
- Purity:
- :≥99.5%&99.9%
- Supply Ability:
- 500MT

US $0.00-0.00/kg2025-09-10
- CAS:
- 2420-87-3
- Min. Order:
- 10kg
- Purity:
- 99%min
- Supply Ability:
- 10 tons