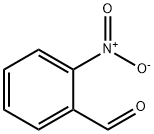
2-Nitrobenzaldehyde: Key Properties and Applications in Analytical Chemistry
General Description
2-Nitrobenzaldehyde is a yellow crystalline aromatic aldehyde with a molecular formula of C7H5N1O3, known for its unique physical, chemical, and biological properties. It acts as an effective antioxidant by suppressing singlet oxygen generation, which enhances its applications in material protection and stability, particularly in food and photonic materials. Additionally, 2-nitrobenzaldehyde serves as a vital actinometer in analytical chemistry, specifically for measuring ultraviolet light doses in drug photostability testing. Its versatility across various illumination sources ensures accurate assessments of pharmaceutical stability, contributing significantly to the rigor and efficiency of laboratory practices.

Figure 1. 2-Nitrobenzaldehyde
Properties
Physical and Chemical Properties
2-Nitrobenzaldehyde is a yellow crystalline compound classified as an aromatic aldehyde with the molecular formula C7H5N1O3. It exhibits distinct physical properties, including a melting point of approximately 95 degrees Celsius and a boiling point of around 212 degrees Celsius. This compound is soluble in organic solvents such as ethanol and acetone, but it has limited solubility in water. The molecular structure of 2-nitrobenzaldehyde contains a nitro group attached to a benzaldehyde, which imparts unique chemical properties that facilitate its application in various fields, particularly in organic synthesis and as a reagent in chemical reactions. The presence of the nitro group not only influences the compound's stability but also enhances its reactivity in electrophilic aromatic substitution reactions. 1
Biological Activity and Antioxidant Properties
In addition to its physical and chemical characteristics, 2-nitrobenzaldehyde possesses significant biological activity, particularly in its role as an antioxidant. Research indicates that 2-nitrobenzaldehyde effectively suppresses the generation of singlet oxygen, a reactive oxygen species, when exposed to ultraviolet and visible light in aerobic environments. This suppressing effect is crucial in mitigating oxidative stress in biological systems, thereby preventing cellular damage. The comparative analysis of various nitrobenzaldehyde isomers has demonstrated that 2-nitrobenzaldehyde outperforms other antioxidants, such as butylated hydroxytoluene and butylated hydroxyanisole, in protecting materials from singlet oxygen formation. The antioxidants effectiveness of 2-nitrobenzaldehyde establishes its potential application in the food industry and polymer science, where oxidative degradation poses significant challenges. 2
Implications in Material Protection
The notable protective influence of 2-nitrobenzaldehyde extends beyond its antioxidant capacities, as it substantially reduces the oxidation of fatty acids and the degradation of dye photosensitizers. The compound's ability to minimize these processes suggests that the presence of 2-nitrobenzaldehyde can enhance the stability and longevity of food products and photonic materials exposed to light. Furthermore, 2-nitrobenzaldehyde operates effectively across a broad spectral range, specifically from 300 nanometers to 575 nanometers, which corresponds with significant wavelengths of ambient solar radiation. Therefore, the integration of 2-nitrobenzaldehyde in material formulations could serve as a strategic approach to preserve the quality and effectiveness of sensitive products, ensuring their robust performance in practical applications, particularly where light exposure is a critical factor. 2
Applications in Analytical Chemistry
2-Nitrobenzaldehyde as a Photochemical Actinometer
2-Nitrobenzaldehyde serves a vital role in analytical chemistry, particularly in photostability testing for pharmaceuticals. Its unique properties allow it to act as an effective chemical actinometer for measuring ultraviolet A and ultraviolet B light doses, specifically within the 290 to 400 nanometer range. The photochemical titration method utilizing 2-nitrobenzaldehyde relies on its predictable photolytic behavior in aqueous solutions, enabling accurate and reliable assessments of light exposure during drug stability tests. This method is user-friendly, making it accessible for chemical technicians who can implement it with common reagents, thus promoting efficiency in laboratory practices.
Versatility in Various Illumination Sources
The versatility of 2-nitrobenzaldehyde is further highlighted by its compatibility with various illumination sources, including xenon arc lamps commonly utilized in drug photostability testing. As analytical chemistry places significant emphasis on accuracy, the employment of 2-nitrobenzaldehyde in measuring light doses ensures precise evaluations of pharmaceutical formulations' stability under light exposure. Its adaptability implies that researchers can modify the method for different light sources while maintaining the effectiveness of 2-nitrobenzaldehyde as an actinometer, thus enhancing the scope of photostability assessment. Overall, the applications of 2-nitrobenzaldehyde in analytical chemistry not only streamline testing processes but also contribute to the rigorous evaluation of drug formulations, ensuring their safety and efficacy. 3
References:
[1] MAHDI HAJIMOHAMMADI. Suppressing Effect of 2-Nitrobenzaldehyde on Singlet Oxygen Generation, Fatty Acid Photooxidation, and Dye-Sensitizer Degradation.[C]//7 12. 2018. DOI:10.3390/antiox7120194.[2] JOHN M. ALLEN S W B Sandra K Allen. 2-Nitrobenzaldehyde: a convenient UV-A and UV-B chemical actinometer for drug photostability testing[J]. Journal of pharmaceutical and biomedical analysis, 2000, 24 2: 167-334. DOI:10.1016/S0731-7085(00)00423-4.
Related articles And Qustion
See also
Lastest Price from 2-Nitrobenzaldehyde manufacturers
US $0.00/kg2025-11-21
- CAS:
- 552-89-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs
US $0.00-0.00/kg2025-11-20
- CAS:
- 552-89-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons