2-Amino-N-(2,2,2-trifluoroethyl)acetamide: Properties, Applications in Preparation of Fluralaner and Its Green Preparation
General Description
2-Amino-N-(2,2,2-trifluoroethyl)acetamide is a versatile acetamide derivative crucial in organic synthesis and medicinal chemistry. Its trifluoroethyl group enhances stability and alters biological activity, impacting properties like solubility and lipophilicity. This compound serves as a key intermediate in creating complex organic molecules and is vital in drug development. In the synthesis of fluralaner, an anthelmintic agent, it plays a pivotal role, offering advantages such as high purity and efficacy. Its green preparation method, involving easily accessible starting materials and environmentally friendly conditions, showcases its potential for sustainable industrial-scale production. Overall, 2-Amino-N-(2,2,2-trifluoroethyl)acetamide is a valuable tool with diverse applications in pharmaceuticals and organic chemistry.

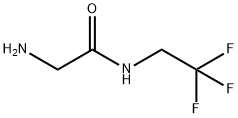
Figure 1. 2-Amino-N-(2,2,2-trifluoroethyl)acetamide
Properties
2-Amino-N-(2,2,2-trifluoroethyl)acetamide is a synthetic organic compound classified as an acetamide derivative. Its chemical structure comprises an amino group bonded to an acetyl moiety, with the acetyl group further linked to a trifluoroethyl substituent. This trifluoroethyl group imparts distinctive characteristics to the molecule, enhancing its stability and altering its biological activity. Primarily utilized as an intermediate compound in organic synthesis, it serves as a foundational component in the creation of more complex organic molecules. Additionally, it finds application in medicinal chemistry as a building block for drug development. The amide functionality of 2-Amino-N-(2,2,2-trifluoroethyl)acetamide enables it to engage in various chemical reactions, facilitating the introduction of diverse functional groups and the modulation of biological properties. Moreover, the presence of the trifluoroethyl substituent influences important physicochemical properties such as solubility, lipophilicity, and metabolic stability. These properties, in turn, impact the compound's pharmacokinetic behavior, affecting its absorption, distribution, metabolism, and excretion within biological systems. In summary, 2-Amino-N-(2,2,2-trifluoroethyl)acetamide serves as a valuable tool in both organic synthesis and medicinal chemistry due to its versatility and tunable properties. Its ability to undergo chemical transformations and modulate biological activity makes it an essential component in the development of novel organic compounds and pharmaceutical agents. 1
Applications in preparation of fluralaner
The synthesis of fluralaner, an isoxazoline anthelmintic compound, involves the utilization of 2-Amino-N-(2,2,2-trifluoroethyl)acetamide as a key component. In the condensation step, this compound reacts with the hydrolyzate and a condensing agent in a solvent medium, resulting in the formation of fluralaner under thermal conditions. 2-Amino-N-(2,2,2-trifluoroethyl)acetamide plays a critical role in the structure and function of fluralaner, which is highly effective against various parasites in animals. The synthesis method offers advantages such as short reaction times, mild conditions, and ease of operation, leading to high-purity fluralaner with an overall yield of 85% to 90%. The final product exhibits exceptional purity, exceeding 99.5%, with minimal impurities (<0.1%), ensuring the safety and efficacy of fluralaner as an anthelmintic agent in veterinary medicine. In conclusion, the incorporation of 2-Amino-N-(2,2,2-trifluoroethyl)acetamide in the synthesis of fluralaner underscores its significance in producing a potent anthelmintic compound with wide-ranging applications in animal health and agriculture. 2
Green preparation
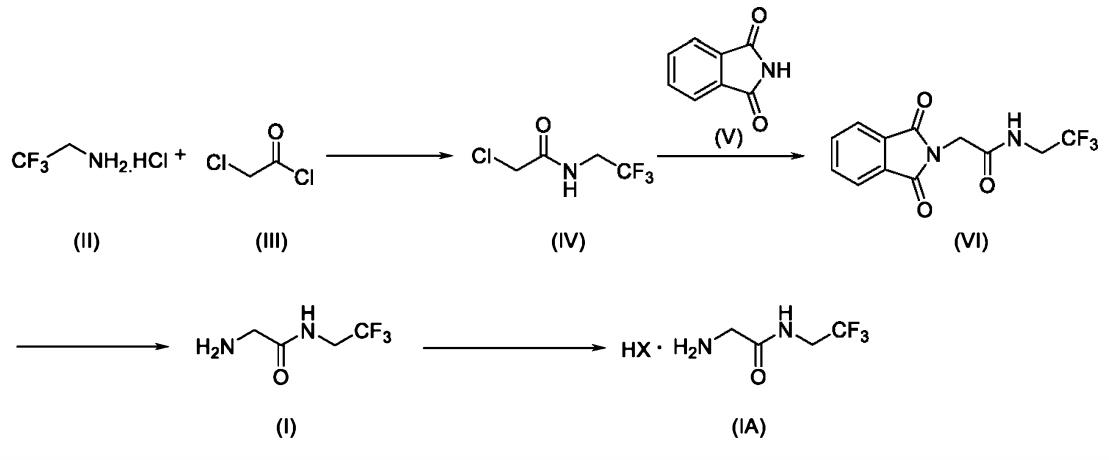
The green preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide involves a straightforward method utilizing easily accessible starting materials and environmentally friendly reaction conditions. The process can be outlined as follows: Firstly, BOC-glycine is reacted with trifluoroethylamine in the presence of carbonyldiimidazole and a solvent at room temperature. This step yields 2,2,2-trifluoroethyl 2-(tert-butoxycarbonylamino)acetate. Secondly, the product from the first step is reacted with hydrochloric acid to obtain 2,2,2-trifluoroethyl 2-(tert-butoxycarbonylamino)acetate hydrochloride. Finally, the product from the second step is subjected to a reaction in the presence of ammonia gas, with pH adjustment, to yield 2-amino-N-(2,2,2-trifluoroethyl)acetamide. The advantages of this method lie in the simplicity and mildness of the reaction conditions, as well as the accessibility and affordability of the starting materials. Additionally, the preparation route exhibits minimal environmental impact, aligning with the principles of green chemistry. The yield is stable, reaching 82%, and the process is suitable for industrial-scale production. Overall, this method represents an efficient and sustainable approach to synthesizing 2-amino-N-(2,2,2-trifluoroethyl)acetamide, demonstrating the potential for practical application in various industrial settings. 3
Reference
1. 2-Amino-N-(2,2,2-trifluoroethyl)acetamide. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 24705143.
2. Zhu JM, Zhang JY, Wang XC. Method for preparation of isoxazoline anthelmintic fluralaner. 2022; Patent Number: CN113896690.
3. Song X. Green preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide. 2023; Patent Number: CN116102446.
Related articles And Qustion

US $3.00-9.00/KG2025-06-28
- CAS:
- 359821-38-8
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available