2-(3-(4-hydroxyphenyl)propanamido)benzoic acid: Preparation Method, Natural Sources and Potential in Skin Therapy
General Description
The synthesis of 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid involves esterification and amidation processes, yielding high conversion rates and up to 92% yield. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is naturally found in oats and has garnered attention for its diverse biological activities, including antioxidant, anti-inflammatory, and potential anti-cancer properties. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is known to reduce cholesterol levels and protect against cardiovascular diseases. Additionally, it has shown promise in skincare and cosmetic products due to its skin-soothing, protective, and anti-inflammatory effects. In skin therapy, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid has demonstrated the ability to inhibit UV-induced skin damage and aging by regulating ROS production, MMP expression, and key signaling pathways. These findings highlight its potential as a preventive agent against UV-induced skin photoaging, making it a valuable candidate for developing skincare products aimed at protecting the skin and preventing premature aging.

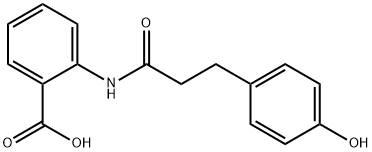
Figure 1. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
Preparation method
The preparation method of 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid involves a series of steps starting from p-hydroxyphenylpropionic acid through esterification and amidation processes. Initially, p-hydroxyphenylpropionic acid is reacted with a lower alcohol (such as methanol) in the presence of a catalyst like concentrated sulfuric acid to form p-hydroxyphenylpropionate. Subsequently, this compound undergoes amidation with anthranilic acid at 130-140°C using a catalyst like trimethylaluminum in a suitable solvent like dimethyl sulfoxide. The reaction mixture is then quenched to yield crude 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid. Lastly, recrystallization and drying steps are employed to obtain the final product. This method boasts improved raw material conversion rates, a short process with high yield reaching up to 92%, making it suitable for large-scale industrial production. The optimized conditions and precise control of reaction parameters contribute to the efficiency and success of this synthesis route. 1
Natural sources
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is a naturally occurring compound found in oats (Avena sativa). It is classified as a hydroxycinnamic acid amide derivative and is known for its various biological activities. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is primarily derived from the active ingredients present in oats. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid has gained significant attention due to its potential health benefits. It exhibits antioxidant, anti-inflammatory, and antiproliferative properties. Studies have indicated its role in reducing cholesterol levels, protecting against cardiovascular diseases, and possessing anti-cancer properties. The natural source of 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid, oats, has long been recognized for its nutritional value and health-promoting effects. As a result, it is commonly used in skincare and cosmetic products for its potential skin-soothing and protective properties. Additionally, its anti-inflammatory effects make it a valuable ingredient in formulations targeting sensitive or irritated skin. Overall, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is a naturally occurring compound derived from oats, offering a wide range of potential health benefits and serving as a valuable ingredient in various applications, including skincare and cosmetic products. 2
Potential in skin therapy
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid shows promising potential in skin therapy due to its ability to prevent UV-induced skin damage and aging. In a study on human dermal fibroblasts, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid was found to inhibit the production of reactive oxygen species (ROS) and the expression of matrix metalloproteinases (MMPs) - specifically MMP-1 and MMP-3 - induced by UVB radiation. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid, a synthetic analog of avenanthramide found in oats, has been known for its anti-inflammatory, anti-atherosclerotic, and antioxidant properties. This study further elucidated its role in protecting against photoaging caused by UV exposure. By inhibiting ROS generation and MMP expression, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid effectively countered the damaging effects of UV radiation on skin cells. Moreover, it was observed to regulate key signaling pathways involved in UV-induced skin damage, including MAPK/NF-κB and AP-1. By blocking these pathways, it can mitigate the harmful effects of UV radiation on skin cells, suggesting its potential as a preventive agent against UV-induced skin photoaging. Overall, the findings suggest that 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid could be a valuable candidate for developing skincare products aimed at protecting the skin from the detrimental effects of UV exposure and preventing premature skin aging. 3
Reference
1. Preparation method of 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid from p-hydroxyphenylpropionic acid via esterification and amidation. 2022. Patent Number: CN114478296.
2. Lee YR, Noh EM, Oh HJ, et al. Dihydroavenanthramide D inhibits human breast cancer cell invasion through suppression of MMP-9 expression. Biochem Biophys Res Commun. 2011;405(4):552-557.
3. Kim JM, Noh EM, Kwon KB, et al. Dihydroavenanthramide D prevents UV-irradiated generation of reactive oxygen species and expression of matrix metalloproteinase-1 and -3 in human dermal fibroblasts. Exp Dermatol. 2013;22(11):759-761.
Related articles And Qustion
See also
Lastest Price from 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid manufacturers
US $0.00/kg2025-09-11
- CAS:
- 697235-49-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 2000kgs
US $0.00-0.00/kg2025-08-20
- CAS:
- 697235-49-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1