2-(2-Bromophenyl)pyrrolidine: Overview and Applications in Medicinal Chemistry
General Description
2-(2-Bromophenyl)pyrrolidine, a versatile compound with a brominated phenyl substituent and a stable pyrrolidine ring, holds significant promise in medicinal chemistry. Its role in the synthesis of ABAD inhibitors showcases its potential in treating Alzheimer's and cancer by modulating enzyme activity effectively. Additionally, its involvement in preparing Bcl-2 inhibitors highlights its importance in addressing diseases related to Bcl-2 dysregulation, such as cancers and autoimmune disorders. The compounds derived from 2-(2-Bromophenyl)pyrrolidine exhibit potent inhibitory effects on target proteins, suggesting a novel class of inhibitors with potential efficacy against resistant mutations. Overall, the application of 2-(2-Bromophenyl)pyrrolidine in drug development signifies a crucial advancement in creating therapeutics for various debilitating conditions.

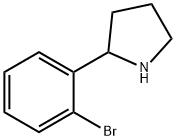
Figure 1. 2-(2-Bromophenyl)pyrrolidine
Overview
2-(2-Bromophenyl)pyrrolidine is a synthetic organic compound within the pyrrolidine family, distinguished by its brominated phenyl substituent. This structural feature confers unique chemical reactivity, facilitating further derivatization and modification. Moreover, the presence of the pyrrolidine ring enhances the compound's stability and bioactivity, rendering it a promising candidate for pharmaceutical purposes. Its synthesis typically involves organic reactions like substitution and cyclization, resulting in a pure, well-defined product. Given its distinctive properties, 2-(2-Bromophenyl)pyrrolidine serves as a valuable building block in organic chemistry, offering potential applications in drug discovery and material science. Its bromine moiety enhances its reactivity, while the pyrrolidine ring contributes to its stability and bioactivity, making 2-(2-Bromophenyl)pyrrolidine an intriguing compound for various research and development endeavors. 1
Applications in Medicinal Chemistry
ABAD inhibitors
The application of 2-(2-Bromophenyl)pyrrolidine in the preparation of ABAD inhibitors is a significant development in the field of medicinal chemistry, particularly in the treatment of diseases such as Alzheimer's and cancer. Amyloid Binding Alcohol Dehydrogenase (ABAD) inhibitors I and II offer promising therapeutic potential. These inhibitors are characterized by their structural diversity, with functional groups that contribute to their pharmacological activity. 2-(2-Bromophenyl)pyrrolidine, as a key component in the synthesis of these inhibitors, plays a crucial role in enhancing their ABAD inhibitory properties. Its specific structure and functional groups enable it to interact effectively with the target enzyme, NAD+H (NADH), thereby modulating its activity non-competitively. The non-competitive inhibition of ABAD by these inhibitors highlights their potential therapeutic relevance in the treatment and prevention of diseases such as Alzheimer's and cancer. The ability to disrupt ABAD activity holds promise for mitigating the pathological processes associated with these diseases, making the development of effective ABAD inhibitors a significant focus in drug discovery. The evaluation of these compounds for their ABAD inhibitory activities further underscores their potential as valuable candidates for medicinal use. In conclusion, the application of 2-(2-Bromophenyl)pyrrolidine in the synthesis of ABAD inhibitors represents a crucial advancement in drug development, offering new avenues for the treatment and management of debilitating diseases like Alzheimer's and cancer. 2
Bcl-2 inhibitors
2-(2-Bromophenyl)pyrrolidine plays a crucial role in the preparation of N-(phenylsulfonyl)benzamides, which are utilized as Bcl-2 inhibitors. These inhibitors are significant in treating diseases associated with undesirable Bcl-2 activity, including dysregulated apoptotic diseases such as cancers and autoimmune diseases. The process involves a multi-step synthesis starting from (S)-2-(2-bromophenyl)pyrrolidine. Various reagents like cyclopropylboronic acid, tert-Bu 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate, 1H-pyrrolo[2,3-b]pyridin-5-ol, and 4-[[(4-fluorotetrahydro-2H-pyran-4-yl)methyl]amino]-3-nitrobenzenesulfonamide are employed in the synthesis process. The resulting compounds are then tested for their ability to block the Bcl-2/Bcl-X protein interaction and regulate apoptosis in RS4 acute lymphoblastic leukemia. Some of these compounds exhibit significantly higher potency and selectivity while demonstrating lower inhibition of CYP2C9, indicating potential for improved efficacy and reduced risk of drug-drug interactions. Moreover, select compounds show inhibitory activity against both Bcl-2 wild type and Bcl-2-G101V mutation type, suggesting a novel class of Bcl-2 inhibitors with potential effectiveness against resistant mutations. In summary, the application of 2-(2-Bromophenyl)pyrrolidine in the synthesis of Bcl-2 inhibitors represents a promising avenue for the development of therapeutics targeting Bcl-2-related diseases. 3
Reference
1. 2-(2-Bromophenyl)pyrrolidine. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 3299348.
2. Aitken L, Gunn-Moore F, Smith TK. Preparation of heterocyclic compounds as ABAD inhibitors. 2023; Patent Number: WO2023026060.
3. Guo YH, Xue H, Wang ZW, Sun HZ. Preparation of N-(phenylsulfonyl)benzamides as Bcl-2 inhibitors. 2019; Patent Number: WO2019210828.