2,2'-Bis(trifluoromethyl)benzidine: Production and Applications in Synthesis of Copolymers
General Description
A new industrial process has been developed for the synthesis of 2,2'-Bis(trifluoromethyl)benzidine with a high yield of 99%. This compound plays a crucial role in the development of colorless polyimide copolymers and poly(ester imide) copolymers, which have various applications in optical and optoelectronic fields and as dielectric substrate materials for high-frequency flexible printed circuit boards, respectively. The incorporation of 2,2'-Bis(trifluoromethyl)benzidine in these copolymers results in unique properties such as improved solubility, increased thermal expansion coefficients, high glass transition temperatures, and excellent dielectric properties. Overall, this new synthetic route offers a reliable and efficient method for the production of 2,2'-Bis(trifluoromethyl)benzidine, and these copolymers show great promise in advanced electronic applications requiring reliable performance under demanding conditions.

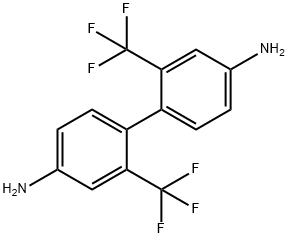
Figure 1. 2,2'-Bis(trifluoromethyl)benzidine
Production
A new industrial process has been developed for the synthesis of 2,2'-Bis(trifluoromethyl)benzidine with a high yield of 99%. The production involves three steps. In the first step, a Grignard reagent derived from 1-chloro-2-(trifluoromethyl)benzene reacts with iron chloride(III) in the presence of an oxidizing agent. This reaction leads to the formation of 2,2'-Bis(trifluoromethyl)biphenyl. The second step involves nitration of the biphenyl obtained from the first step. This nitration reaction converts it into 2,2'-Bis(trifluoromethyl)-4,4'-dinitrobiphenyl. Finally, in the third step, the dinitrobiphenyl undergoes reduction using hydrogen over Pd/C catalyst to yield the desired product, 2,2'-Bis(trifluoromethyl)benzidine. The entire process is carried out under specific conditions. The reaction is conducted at a pressure of 0.3 MPa and at room temperature initially, followed by heating to 55 °C for one hour, and then maintaining the temperature at 55 °C for another hour. This new synthetic route has been successfully demonstrated on an industrial scale, offering a reliable and efficient method for the production of 2,2'-Bis(trifluoromethyl)benzidine. 1
Applications in synthesis of copolymers
Colorless polyimide copolymers
2,2'-Bis(trifluoromethyl)benzidine plays a crucial role in the development of colorless polyimide copolymers with various applications. These copolymers are synthesized using TFMB along with biphenyltetracarboxylic dianhydrides through either one-step or two-step methods. The incorporation of 2,2'-Bis(trifluoromethyl)benzidine in these copolymers results in unique properties and characteristics. The d-spacing values of these copolymers range from 5.7 to 6.7 Å, with an increase observed as the 3,3'-BPDA/2,2'-Bis(trifluoromethyl)benzidine contents rise. This increase in d-spacing is accompanied by a decrease in the intensity of π-π stacking interactions. Moreover, the solubility of the copolymers improves with higher 3,3'-BPDA/2,2'-Bis(trifluoromethyl)benzidine contents. Additionally, the coefficients of thermal expansion and glass transition temperatures of the copolymers increase within the range of 15 to 68 ppm K−1 and 300 to 359 °C, respectively, with increasing 3,3'-BPDA/2,2'-Bis(trifluoromethyl)benzidine contents. Furthermore, these copolymers exhibit excellent optical transparency, making them suitable for applications in optical and optoelectronic fields. Specifically, CPI-4 and CPI-5, containing 40 mol% and 20 mol% of 3,3'-BPDA/2,2'-Bis(trifluoromethyl)benzidine contents, demonstrate a well-balanced combination of solution processability and physical properties. Overall, the unique properties of these colorless polyimide copolymers make them promising materials for various industrial applications. 2
Poly(ester imide) copolymers
2,2'-Bis(trifluoromethyl)benzidine plays a crucial role in the synthesis and properties of poly(ester imide) (PEsI) copolymers, which have shown great potential in various applications, particularly as dielectric substrate materials for high-frequency flexible printed circuit boards. The incorporation of 2,2'-Bis(trifluoromethyl)benzidine in PEsI copolymers leads to significant changes in their properties. For instance, the increase of 2,2'-Bis(trifluoromethyl)benzidine contents results in wider interchain distances within the polymers, affecting their thermal and mechanical characteristics. The copolymers exhibit high glass transition temperatures (Tg) ranging from 445 to 455°C, making them suitable for high-temperature applications. Additionally, the coefficients of thermal expansion (CTE) and mechanical properties such as modulus, tensile strength, and elongation-at-break are influenced by the 2,2'-Bis(trifluoromethyl)benzidine content, offering a range of options for tailoring the material properties to specific requirements. Furthermore, the PEsI copolymers demonstrate excellent dielectric properties, including a low dielectric constant and dissipation factor at high frequencies, making them ideal candidates for use in next-generation flexible printed circuit boards. With their unique combination of thermal, mechanical, and electrical properties, these copolymers show great promise in advanced electronic applications requiring reliable performance under demanding conditions. 3
Reference
1. Jiro N, Tatsuhiro N. Homocoupling of a Grignard Reagent toward the Synthesis of 2,2'-Bis(trifluoromethyl)-4,4'-diaminobiphenyl. Organic Process Research & Development, 2021, 25(11): 2442-2446.
2. Xu YZ. Colorless polyimide copolymers derived from isomeric biphenyltetracarboxylic dianhydrides and 2,2'-bis(trifluoromethyl)benzidine. European Polymer Journal, 2023, 193: 112099.
3. Zheng H, Gong D, Pang Y, et al. Synthesis and properties of poly(ester imide) copolymers from 3,3',4,4'-biphenyltetracarboxylic dianhydride, 2,2'-bis(trifluoromethyl)benzidine, and 4-aminophenyl-4'-aminobenzoate. Journal of Applied Polymer Science, 2024, 21: 1.
Related articles And Qustion
Lastest Price from 2,2'-Bis(trifluoromethyl)benzidine manufacturers
US $0.00-0.00/KG2025-11-12
- CAS:
- 341-58-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00/kg2025-10-24
- CAS:
- 341-58-2
- Min. Order:
- 1kg
- Purity:
- ≥99.9%HPLC
- Supply Ability:
- 200MT