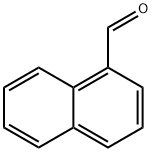
1-Naphthaldehyde: Aromatic Aldehyde Intermediate
1-Naphthaldehyde is an important aromatic aldehyde organic compound. It appears as a pale yellow to yellowish-green crystalline powder or liquid. It is readily soluble in organic solvents such as ethanol, diethyl ether, and benzene, and slightly soluble in water. Regarding preparation, 1-naphthaldehyde can be synthesised via multiple methods. Common routes include the gas-phase oxidation of 1-methylnaphthalene and the acetalisation reaction of naphthalene with carbon monoxide. Among these, the acetalisation reaction is widely employed in industrial production due to its high selectivity and stable yield. As a key intermediate, 1-naphthaldehyde finds extensive applications in organic synthesis, serving in the production of dyes, fluorescent whitening agents, pharmaceutical intermediates, and fragrances.

The ortho photocycloaddition of 1-Naphthaldehyde and 2-naphthaldehyde
Upon direct excitation, 1-naphthaldehyde and 2-naphthaldehyde undergo photochemical transformations at the aldehyde carbonyl group. Like for many other aromatic aldehydes, the typical reaction products are oxetanes (Paternò–Büchi reaction), alcohols (photoreduction) or carbonyl addition products (photoaddition). The reaction of 1-naphthaldehyde (1) in 2,3-dihydrofuran for example has been reported to deliver oxetane 3 in a yield of 55%. The reactivity pattern is determined by the nπ* character of the reactive excited states (singlet or triplet) or it is due to electron transfer pathways which generate a radical anion by reduction of the carbonyl group. In recent work, the influence of Lewis acids on photochemical reactions has been studied with a focus on enantioselective transformations. However, it was also noted that the Lewis acid modifies other selectivity parameters. We hypothesized that by Lewis acid coordination to the carbonyl group of 1-Naphthaldehyde their typical photochemical behaviour may change because the lowest excited states are of ππ* character. This speculation was supported by previous experiments on photoinduced electron transfer (PET) reactions of silanes to naphthaldehydes. In the absence of a Lewis acid the aldehydes are not sufficiently reactive to undergo a PET with silanes such as benzyltrimethylsilane (TMSBn).[2]
In the context of these reactions, Fukuzumi and co-workers studied the photophysical properties of Lewis acid [Mg(ClO4)2 or Sc(OTf)3] complexes with 1-Naphthaldehyde and 2-naphthaldehyde in great detail. While the enhanced reactivity in the reaction with silanes was ascribed to the higher redox potential of the photoexcited Lewis acid complex, it was also concluded that “the π,π* excited state becomes the lowest excited state in the Mg(ClO4)2 complex as compared with the lowest n,π* triplet excited state in the uncomplexed carbonyl compound”. In the present study, we have compared the photochemical reactivity of 1-Naphthaldehyde and 2-naphthaldehyde towards a typical olefin component (2,3-dimethyl-2-butene) in the absence and in the presence of catalytic amounts of strong Lewis acids. The Lewis acids induced a complete reversal of the type selectivity from carbonyl reactions in the absence of a Lewis acid to reactions at the C1/C2 double bond of the aromatic core. In summary, we have found that naphthaldehydes change their photochemical reactivity pattern dramatically in the presence of EtAlCl2 or AlBr3 as Lewis acids. The site of reactivity is shifted from the carbonyl group to the C1/C2 arene double bond where an initial ortho photocycloaddition with olefins occurs. Consecutive Lewis acid promoted reactions lead to an opening of the cyclobutane ring and to the formation of a C2-alkylated naphthalene in the case of 1-naphthaldehyde and to a formal [3 + 2] cycloaddition product for 2-naphthaldehyde.
Condensation Product of 1-Naphthaldehyde and 3-Aminophenol
Heavy metal ions are highly toxic and their contamination into the water systems cause pollution of the same. Since water pollution is a prominent global threat, so the detection of toxic species has held much research attention.In this paper, we report that the condensation product of 1-naphthaldehyde and 3-aminophenol, (Z)-3-((naphthalen-1-methylene)amino)phenol (L), acts as a fluorescent sensor for Ce3+ by ‘on’ mode and for Cr2O72− ions by ‘off’ mode. DFT calculations have shown that one Ce3+ is bonded to two L via O atoms forming a square planar structure. CrO3 of Cr2O72− ion is bonded to two L via O atoms forming a trigonal bipyramidal structure. The probe has been synthesized by dissolving 1-naphthaldehyde (136 µL, 1 mmol) and 3-aminophenol (0.109 g, 1 mmol) in dichloromethane (10 mL) and by refluxing for 6 h at 50 ºC. After the reaction was complete, the formed precipitate was obtained using rota-evaporator, then was monitored by TLC. The crude product was then purified using column chromatography.[2]
The condensation product between 1-naphthaldehyde and 3-aminophenol can detect Ce3+ by fluorescent “on” mode while Cr2O72− by fluorescent “off” mode. The Ce3+ detection is interference free from Ag+, Al3+, As3+, Ba2+, Ca2+, Cd2+, Ce4+, Co2+, Cr3+, Cr6+, Cu2+, Fe2+, Fe3+, Hg2+, K+, La+, Li+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Zn2+. The limit of detection (LOD) for sensing Ce3+ and Cr2O72− ions are found to be 1.286 × 10–7 M and 6.425 × 10–6 M, respectively. The naked eye detection of Ce3+ under UV lamp due to the development of fluorescence has been possible.
References
[1]Stegbauer S, Jeremias N, Jandl C, Bach T. Reversal of reaction type selectivity by Lewis acid coordination: the ortho photocycloaddition of 1- and 2-naphthaldehyde. Chem Sci. 2019 Jul 29;10(37):8566-8570. doi: 10.1039/c9sc03315g. PMID: 31803430; PMCID: PMC6839505.
[2]Bordoloi, Priyakshi et al. “Condensation Product of 1-Naphthaldehyde and 3-Aminophenol: Fluorescent "on" Probe for Ce3+and "off" Probe for Dichromate (Cr2O72-).” Journal of fluorescence vol. 32,3 (2022): 1189-1198. doi:10.1007/s10895-022-02927-0
You may like
See also
Lastest Price from 1-Naphthaldehyde manufacturers

US $0.00-0.00/KG2025-08-02
- CAS:
- 66-77-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS

US $50.00-10.00/kg2025-07-17
- CAS:
- 66-77-3
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 1000kg