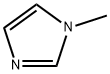
1-Methylimidazole: An Overview
1-Methylimidazole is a colorless to yellow liquid, with an amine-like odor. lt ismiscible with water. lt is a versatile intermediate used as a building block for active ingredients as well as in epoxy curing.
In the traditional medicine industry, imidazoles can be used as antiviral, anti-tumor drug can be used as agricultural bactericide, can be used as the composite material solidification agent, and the electronics processing and fabricating is with novel solderability preservative etc.
Uses
1-Methylimidazole is a derivative of imidazole that is utilized in the manufacture of such classes of items as pharmaceuticals, pesticides, ion-exchange resins, dye intermediates, textile auxiliaries, photographic chemicals, and corrosion inhibitors.
It is also used as a catalyst for manufacturing polyurethanes and a curing agent for epoxy resins. 1-Methylimidazole has been utilized in the synthesis of inorganic complexes, ionic liquids, and catalytic deprotonation reagents. The use of 1-methylimidazole in the preparation of GDP-hexanolamine for the purification of fucosyltransferases has been reported.
Preparation
1-Methylimidazole is prepared mainly by two routes industrially. The main one is acid-catalysed methylation of imidazole by methanol. The second method involves the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine.
See also
Lastest Price from 1-Methylimidazole manufacturers

US $0.00/kg2025-09-02
- CAS:
- 616-47-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $1.00/KG2025-04-21
- CAS:
- 616-47-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt