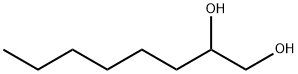
1,2-Octanediol: A Comprehensive Overview
Introduce
1,2-Octanediol is an organic compound. Its appearance is a colorless to light yellow viscous liquid with a special and pleasant aroma. The following is an introduction to the properties, uses, preparation methods, and safety information of 1,2-octanediol.

Figure 1 Characteristics of 1,2-Octanediol
Synthesis of 1,2-Octanediol
Install an electric stirrer, condenser, thermometer, external cold water bath, and a dropper funnel in a four necked flask. Add a measured amount of formic acid, hydrogen peroxide, and catalyst to the flask in sequence, stir vigorously, and mix thoroughly. Under external cooling, add a measured amount of 1-octene dropwise and control the temperature inside the bottle at 35 ℃ -40 ℃ for reaction. After the dropwise addition is complete, keep the reaction at 40 ℃ -45 ℃ for 3-4 hours, and then stir at room temperature for 1-2 hours to obtain a clear and transparent mixed solution. Under high vacuum and reduced pressure, evaporate the clear and transparent mixed solution with water and excess formic acid until only an oily substance remains.
Nature
1,2-Octanediol is soluble in water and most organic solvents, and has good solubility. It is a stable compound that is not easily affected by air and light.
Purpose
1,2-Octanediol has a wide range of applications. It is commonly used as a surfactant, wetting agent, emulsifier, and flame retardant.
Manufacturing method
A commonly used method for preparing 1,2-octanediol is through 1,2-oxidized octane. Firstly, under appropriate conditions, octane is oxidized to form 1,2-octanal, which is then reduced to 1,2-octanediol using a reducing agent.
Environmental degradation pathways of 1,2-Octanediol
Biodegradation
Biodegradation refers to the process in which microorganisms break down organic compounds into carbon dioxide, water, and other simple inorganic substances through metabolic processes. For 1,2-Octanediol, there may be specific microorganisms that can utilize it as a carbon source and energy source to degrade it. For example, certain bacteria and fungi may have the ability to degrade alcohol compounds.
photolysis
Photolysis refers to the process in which organic compounds undergo chemical reactions and decompose under sunlight by absorbing ultraviolet or other wavelengths of light energy. Although the molecular structure of 1,2-Octanediol is relatively simple, it may still be affected by photolysis in the environment, especially in aquatic environments.
Chemical degradation
Chemical degradation refers to the process in which organic compounds undergo chemical reactions and decompose under physical and chemical conditions, such as high temperature, high pressure, or the action of specific chemical reagents. For 1,2-Octanediol, degradation may occur under specific chemical conditions, such as the presence of strong acids, strong bases, or oxidants.
Security information
The general use of 1,2-octanediol is safe, but relevant safety measures must still be followed during use. It is a substance that is less irritating to the eyes and skin, but excessive exposure may cause reversible irritation. When handling, direct contact with eyes and skin should be avoided. When storing and using, it should be kept away from sources of fire and high temperature environments to prevent fires and explosions. Proper operating procedures should be followed and stored properly in appropriate containers.
Toxicity of 1,2-Octanediol
Inhalation risk
When 1,2-Octanediol exists in gas or mist form, it may pose a risk of inhalation. When handling this substance, attention should be paid to providing a good ventilation system or wearing appropriate protective masks.
Eye contact
If 1,2-Octanediol accidentally splashes into the eyes, immediately rinse with plenty of water for at least 15 minutes and seek medical attention as soon as possible.
Skin contact
Although specific skin toxicity data was not explicitly provided in the search results, considering its application in cosmetics, it can be inferred that its direct toxicity to the skin is relatively low. However, prolonged or high concentration exposure may still cause irritation or other adverse reactions.
environmental effect
Under appropriate treatment, 1,2-Octanediol will not cause serious pollution to the environment. Users should follow local and national waste disposal regulations to properly handle and dispose of this compound.
![Article illustration]() Reference
Reference
[1] Burgess I F, Lee P N, Kay K, et al. 1, 2-octanediol, a novel surfactant, for treating head louse infestation: identification of activity, formulation, and randomised, controlled trials[J]. PLoS One, 2012, 7(4): e35419.
[2] Chen L S, Mantovani S M, de Oliveira L G, et al. 1, 2-Octanediol deracemization by stereoinversion using whole cells[J]. Journal of Molecular Catalysis B: Enzymatic, 2008, 54(1-2): 50-54.
References:
[1] IAN F BURGESS. 1,2-Octanediol, a novel surfactant, for treating head louse infestation: identification of activity, formulation, and randomised, controlled trials.[J]. ACS Applied Bio Materials, 2012. DOI:10.1371/journal.pone.0035419.[2] LU S. CHEN . 1,2-Octanediol deracemization by stereoinversion using whole cells[J]. Journal of Molecular Catalysis B-enzymatic, 2008, 54 1: 1-60. DOI:10.1016/j.molcatb.2007.11.022.
You may like
Related articles And Qustion
See also
Lastest Price from 1,2-Octanediol manufacturers

US $100.00-80.00/KG2025-07-17
- CAS:
- 1117-86-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000000

US $1.00/PCS2025-04-21
- CAS:
- 1117-86-8
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt