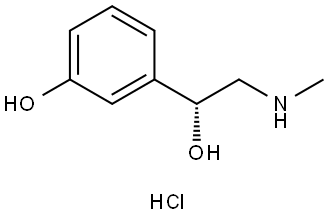
(R)-Phenylephrine Hydrochlorid: Uses and Instructions
Introduction
(R)-Phenylephrine Hydrochlorid is a decongestant. Decongestants have been used to treat nasal congestion (“stuffy nose”) caused by colds or allergies. (R)-Phenylephrine Hydrochlorid can be found in different products, including Sudafed PE. (R)-Phenylephrine Hydrochlorid has also been used to treat temporary low blood pressure caused by anesthesia used during surgeries. Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. Some decongestants or other over-the-counter medications might have multiple active ingredients. Be sure to look at the ingredients on your medications.[1]

Efficacy and safety of oral (R)-Phenylephrine Hydrochlorid
Based on 8 unpublished studies that included 138 patients, (R)-Phenylephrine Hydrochlorid 10 mg did not affect NAR more than placebo the mean maximal difference in relative change from baseline between it and placebo was 10.1% (95% CI -3.8% to 23.9%). Eight unpublished studies on (R)-Phenylephrine Hydrochlorid 25 mg showed a significant reduction of maximal NAR compared with placebo of 27.6% (95% CI 17.5% to 37.7%). There was significant heterogeneity among the studies included in this analysis, which was partially attributable to different laboratories and methods used. Patient-reported decongestion was not consistently better for any (R)-Phenylephrine Hydrochlorid dose compared with placebo, and NAR was a more sensitive measurement of efficacy. It showed no consistent effect on heart rate or blood pressure for doses of 25 mg or less. There is insufficient evidence that oral (R)-Phenylephrine Hydrochlorid is effective for nonprescription use as a decongestant. The Food and Drug Administration should require additional studies to show the safety and efficacy of (R)-Phenylephrine Hydrochlorid.[2]
Effects of (R)-Phenylephrine Hydrochlorid on systemic and cerebral circulations
Blood pressure monitoring and management is standard practice in acute care settings, including operating theatres, intensive care units and emergency departments. Hypotension is common and, as it is linked to unfavourable outcomes, must be managed carefully. (R)-Phenylephrine Hydrochlorid is rapid-acting, effective, titratable and used commonly to treat hypotension. However, some of its observed effects appear paradoxical, such as increasing blood pressure while decreasing cardiac output, and increasing cerebral blood flow while reducing cerebral tissue oxygen saturation as measured by near-infrared spectroscopy.[3]
The increase in cerebral blood flow in the face of the rise in blood pressure appears to contradict the principle of cerebral autoregulation, which posits that cerebral blood flow should remain stable despite changes in blood pressure. Although recent evidence suggests that an increase in blood pressure, with or without the use of a vasopressor, can lead to a finite degree of increase in cerebral blood flow in subjects having intact cerebral autoregulation, we still need to understand the magnitude of the increase in cerebral blood flow following (R)-Phenylephrine Hydrochlorid treatment, as this has not been reported. The opposite directions of (R)-Phenylephrine Hydrochlorid -induced changes in cardiac output (decrease) and cerebral blood flow (increase) challenge the reported positive correlation between these two variables. The observation that cerebral tissue oxygen saturation decreases when cerebral blood flow increases is puzzling, since one would expect the opposite. These observations may discourage practitioners from using (R)-Phenylephrine Hydrochlorid due to its potential negative impact on cardiac output and cerebral oxygenation.
Scientists conducted a systematic review of the literature reporting (R)-Phenylephrine Hydrochlorid -induced changes in blood pressure, cardiac output, cerebral blood flow and cerebral tissue oxygen saturation as measured by near-infrared spectroscopy in humans. They used the proportion change of the group mean values reported by the original studies in our analysis. (R)-Phenylephrine Hydrochlorid elevates blood pressure whilst concurrently inducing a reduction in cardiac output. Furthermore, despite increasing cerebral blood flow, it decreases cerebral tissue oxygen saturation. The extent of (R)-Phenylephrine Hydrochlorid 's influence on cardiac output (r = -0.54 and p = 0.09 in awake humans r = -0.55 and p = 0.007 in anaesthetised humans), cerebral blood flow (r = 0.65 and p = 0.002 in awake humans r = 0.80 and p = 0.003 in anaesthetised humans) and cerebral tissue oxygen saturation (r = -0.72 and p = 0.03 in awake humans r = -0.24 and p = 0.48 in anaesthetised humans) appears closely linked to the magnitude of (R)-Phenylephrine Hydrochlorid -induced blood pressure changes. When comparing the effects of (R)-Phenylephrine Hydrochlorid in awake and anaesthetised humans, they found no evidence of a significant difference in cardiac output, cerebral blood flow or cerebral tissue oxygen saturation. There was also no evidence of a significant difference in effect on systemic and cerebral circulations whether (R)-Phenylephrine Hydrochlorid was given by bolus or infusion. We explore the underlying mechanisms driving the (R)-Phenylephrine Hydrochlorid -induced cardiac output reduction, cerebral blood flow increase and cerebral tissue oxygen saturation decrease. Individualised treatment approaches, close monitoring and consideration of potential risks and benefits remain vital to the safe and effective use of (R)-Phenylephrine Hydrochlorid in acute care.
References
[1] Mother To Baby | Fact Sheets [Internet]. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); 1994-. Phenylephrine. 2024 Sep.
[2] Hatton RC, Winterstein AG, McKelvey RP, Shuster J, Hendeles L. Efficacy and safety of oral phenylephrine: systematic review and meta-analysis. Ann Pharmacother. 2007 Mar;41(3):381-90.
[3] Meng L, Sun Y, Zhao X, Meng DM, Liu Z, Adams DC, McDonagh DL, Rasmussen M. Effects of phenylephrine on systemic and cerebral circulations in humans: a systematic review with mechanistic explanations. Anaesthesia. 2024 Jan;79(1):71-85
Lastest Price from (R)-Phenylephrine Hydrochlorid manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 61-76-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/KG2025-05-06
- CAS:
- 61-76-7
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG

![5807-14-7 1,5,7-Triazabicyclo[4.4.0]dec-5-eneOrganocatalyst superbase catalysis](/NewsImg/2025-02-26/6387617385737524549193443.jpg)