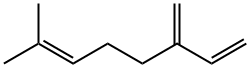
β-Myrcene: A Versatile Monoterpene with Diverse Properties and Production Methods
Myrcene (β-myrcene) is an abundant monoterpene which occurs as a major constituent in many plant species, including hops and cannabis. It is a popular flavouring and aroma agent (food additive) used in the manufacture of food and beverages. This review aims to report on the occurrence, biological and toxicological profile of β-myrcene. The main reported biological properties of β-myrcene—anxiolytic, antioxidant, anti-ageing, anti-inflammatory, analgesic properties—are discussed, with the mechanisms of activity.

Microbial synthesis of plant-based myrcene
An effective one-step biotransformation system for myrcene biosynthesis from geraniol, using a linalool dehydratase isomerase (LDI). The truncated LDI possesses nominal activity that catalyzes the isomerization of geraniol to linalool and the subsequent dehydration to myrcene in anaerobic environment. Geranyl diphosphate (GPP) is thus formed via condensation of IPP with DMADP, by GPP synthase (GPPS). Geranyl diphosphate (GPP) then undergoes hydrolysis catalysed by a prenolpyrophosphatase to form geraniol. Consequently, β-myrcene is produced by the dehydration and isomerization of geraniol.
Industrial Synthesis of Myrcene
β-Pinene is an important starting material for the synthesis of β- myrcene. β-pinene can be isolated from the waste streams of paper mills, by distillation and desulphurisation from crude sulphate turpentine (CST).
References:
[1] XUN WANG . Efficient myrcene production using linalool dehydratase isomerase and rational biochemical process in Escherichia coli[J]. Journal of biotechnology, 2023, 371: 1-50. DOI:10.1016/j.jbiotec.2023.05.008.[2] SHELINI SURENDRAN. Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent?[J]. ACS Applied Energy Materials, 2021. DOI:10.3389/fnut.2021.699666.
You may like
Lastest Price from Myrcene manufacturers

US $45.00-35.00/kg2025-10-31
- CAS:
- 123-35-3
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 50 Tons

US $3.00/kg2025-04-21
- CAS:
- 123-35-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000