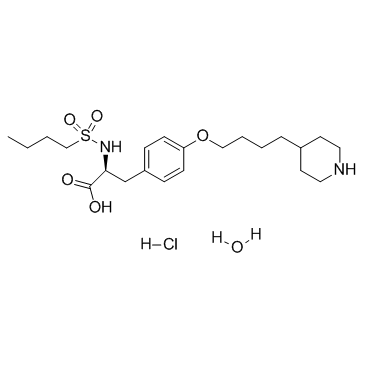
盐酸替罗非班
Tirofiban Hydrochloride
150915-40-5
150915-40-5
询价
5kg
起订
10kg
起订
浙江 更新日期:2021-03-26
产品详情:
公司简介
一般项目:第二类医疗器械销售;针纺织品及原料批发;服装复试批发;日用百货销售;家用电器销售;第一类医疗器械销售;化工产品销售(不含许可类化工产品);五金产品批发;计算机软硬件及辅助设备批发;化妆品批发;消毒剂销售(不含危险化学品);专用化学产品销售(不含危验化学品);个人卫生用品销售;金属材料售;医用口罩批发;日用口罩(非医用)销售;医护人员防护用品批发;卫生用品和一次性使用医疗用品销售;劳动保护用品确售;合成材料销售;饲料添加剂销售;药物检测仪器销售;制药专用设备销售;财务咨询;信息咨询服务(不含许可类信息咨询服务);技术服务、技术开发、技术咨询、技术交流、技术转让、技术推广;染料销售;卫生用品销售;建筑装饰材料销售;电子办公设备销售;针纺识品销售;贸易经纪;销售代理;国内贸易代理(除依法须经批准的项目外,凭营业执照依法自主开展经营活动)。许可项目∶食品经营(销售预包装食品);货物进出口;药品进出口;进出口代理;技术进出口(依法须经批准的项目,经相关部门批批准方可开展经营活动,具体经营项目以审批结果为准)。
| 成立日期 | (23年) |
| 注册资本 | 1000万人民币 |
| 员工人数 | 1-10人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 贸易 |
| 主营行业 | 中间体,医药原料 |
盐酸替罗非班相关厂家报价 更多
-

- 盐酸替罗非班
- 湖北众善奉行医药科技有限公司 VIP
- 2026-01-30
- 询价
-

- 盐酸替罗非班
- 湖北鸿福达生物科技有限公司 VIP
- 2026-01-30
- 询价
-

- 150915-40-5盐酸替罗非班 99% 生产工艺方法 15871722230
- 武汉鼎信通药业有限公司 VIP
- 2026-01-30
- ¥150
-

- 盐酸替罗非班
- 武汉维斯尔曼生物工程有限公司 VIP
- 2026-01-30
- 询价
-

- 盐酸替罗非班—150915-40-5
- 湖北魏氏化学试剂股份有限公司 VIP
- 2026-01-30
- ¥479
-

- 盐酸替罗非班—150915-40-5
- 湖北魏氏化学试剂股份有限公司 VIP
- 2026-01-30
- 询价
-
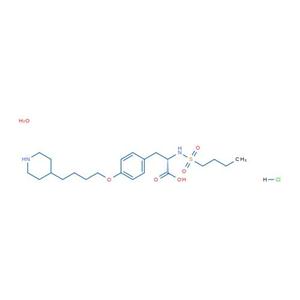
- 盐酸替罗非班
- 中国食品药品检定研究院
- 2026-01-29
- 询价
-

- 99%盐酸替罗非班用途150915-40-5抗血栓用于不稳定性心绞痛或非Q波心肌梗塞
- 武汉维斯尔曼生物工程有限公司 VIP
- 2026-01-26
- ¥300000
-

- 盐酸替罗非班
- 湖北辰心药业有限公司 VIP
- 2026-01-24
- 询价
-
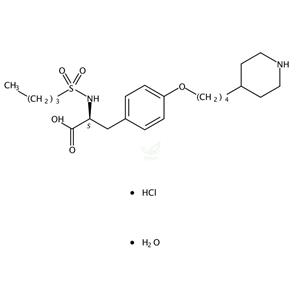
- 盐酸替罗非班 一水合物 150915-40-5
- 成都彼样生物科技有限公司 VIP
- 2026-01-22
- 询价