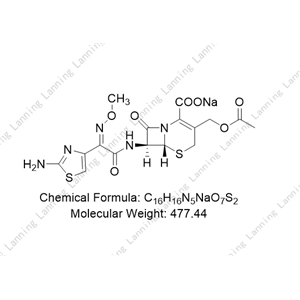
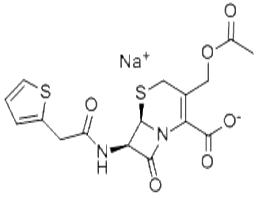
头孢噻吩钠
TEL: 18062666980
【性状】 本品为白色或类白色的结晶性粉末;几乎无臭。
本品在水中易溶,在乙醇中微溶,在氯仿或乙醚中不溶。
比旋度 取本品,精密称定,加水溶解并稀释制成每1ml 中含10mg的溶液,在
25℃时,依法测定(附录Ⅵ E),比旋度为+124°至+134°。
【鉴别】 (1)在含量测定项下记录的色谱图中,供试品主峰的保留时间应与头孢噻吩
对照品主峰的保留时间一致。
(2) 本品的红外光吸收图谱应与对照的图谱(光谱集129 图)一致。
(3) 本品显钠盐的火焰反应(附录Ⅲ)。
【检查】 酸度 取本品,加水制成每1ml中含0.1g的溶液,依法测定(附录Ⅵ H
),pH值应为4.5~7.0 。
溶液的澄清度与颜色 取本品5 份,各0.6g,分别加水5ml 溶解后,溶液应澄清无
色;如显浑浊,与1 号浊度标准液(附录Ⅸ B)比较,均不得更浓;如显色,与黄色或
黄绿色5号标准比色液(附录Ⅸ A法)比较,均不得更深。
吸收度 取本品,加水制成每1ml 中含20μg 的溶液,照分光光度法(附录Ⅳ A),
在237nm 的波长处测定,其吸收度为0.65~0.72。
水分 取本品,照水分测定法(附录Ⅷ M法A)测定,含水分不得过1.2%。
热原 取本品,加灭菌注射用水制成每1ml中含50mg的溶液,依法检查(附录Ⅺ D
),剂量按家兔体重每1Kg 注射1ml ,应符合规定。
无菌 取本品,分别加入100ml 0.9%无菌氯化钠溶液中使溶解,用薄膜过滤法处理后,
依法检查(附录Ⅺ H),应符合规定。
【含量测定】 照高效液相色谱法(附录Ⅴ D)测定。
色谱条件与系统适用性试验 用十八烷基硅烷键合硅胶为填充剂;醋酸溶液(1→100)
-乙腈(3:1)为流动相;检测波长为254nm。调节流速使头孢噻吩峰的保留时间约匀为7分钟。
测定法 取本品适量,精密称定,加水溶解并制成每1ml中约含0.5mg的溶液,取10μl
注入液相色谱仪,记录色谱图;另取头孢噻吩对照品适量,同法测定。按外标法以峰面积计算
出供试品中C16H16N2O6S2的含量。
【类别】 抗生素类药。
【贮藏】 严封,在凉暗干燥处保存。
【制剂】 注射用头孢噻吩钠
195 C16H15N2O6S2 418.43 Cymbalta (6R, 7R) -3 - [(acetyloxy) methyl] -7 - [2 - (2 - thiophene-yl) acetamido]-8 - oxo - sulfur Oxa-1 - azabicyclo [4.2.0] oct-2 - ene - 2 - sodium salt. Calculated on the anhydrous containing Cefalotin (C16H16N2O 6S2) shall not be less than 90.0%. 【Properties】 white or off-white crystalline powder; almost odorless. This product is soluble in water, slightly soluble in ethanol, insoluble in chloroform or ether. Specific rotation to take this product, accurately weighed, dissolved in water and diluted per 1ml contains 10mg solution in Determination in accordance with the law (Appendix Ⅵ E), 25 ℃ Specific rotation +124 ° to +134 °. Identification (1) Determination under-recorded chromatogram, the retention time of the main peak of the test should be Cefalotin Of standard main peak in the same retention time. (2) The product of the infrared absorption spectrum should control spectra (spectral set of 129). (3) This product was sodium salt of the flame reaction (Appendix III). [Check] acidity to take this product, add water containing 0.1 g solution per 1ml, measured according to the law (Appendix VI H ), PH value of 4.5 to 7.0. Clarity and color of solution is to take this product, each 0.6g, 5ml of water dissolved, the solution should be clarified without Color; significant turbidity, compared with the 1st turbidity standard solution (Appendix Ⅸ B), shall be thicker; such as color, with yellow or Yellow-green on the 5th standard colorimetric solution (Appendix Ⅸ A first method), shall not be deeper. Absorbance of this product, the addition of water per 1ml containing 20μg solution, according to spectrophotometry (Appendix Ⅳ A) 237 nm at a wavelength of determination, the absorbance of 0.65 to 0.72. The moisture of this product, measured according to the determination of moisture content (Appendix Ⅷ M and A) on the Law of water containing not more than 1.2%. The pyrogen to take this product, add sterile water for injection containing 50 mg solution per 1ml, according to the law check (Ⅺ D Appendix ), The dose per of 1Kg injection 1 ml of rabbit weight should be required. Sterile to take this product were added to 100ml 0.9% sterile sodium chloride solution to dissolve after treatment with membrane filtration method , Shall comply with the provisions of the law examination (Appendix XI H). 【Assay for high performance liquid chromatography (Appendix Ⅴ D). Determination. Chromatographic conditions and system suitability test together with Octadecylsilanized silica as a filler; acetic acid solution (1 → 100) - Acetonitrile (3:1) as the mobile phase; detection wavelength was 254 nm. The flow rate was adjusted to make Cefalotin peak retention time is approximately uniform for 7 minutes. Take some assay, accurately weighed, dissolved in water and the solution containing 0.5mg per 1ml about, take 10μl Into the liquid chromatograph, record the chromatogram; Another Cefalotin appropriate reference, the same method. Peak area of ??the external standard method Out the test C16H16N2O6S2 content. 【Category】 antibiotic medicines. 【Storage】 strict closure stored in a cool dark dry place. [Preparations] for injection Cephalothin sodium
武汉远城科技发展有限公司位于风景秀丽的首义创新生化工业园,是中南地区大型的专业从事精细化工,香精香料,食品添加剂,医药原料及中间体等产品的研究,开发,生产,经营,销售于一体的高科技化工企业。
公司属中南地区大型的专业从事精细化工,食品添加剂,医药原料及中间体等产品的研究、开发、生产、销售于一体的高科技化工集团公司。
公司固定资产总投资资已达成1亿余元,拥有按GMP标准建造从中试到大生产,设备先进,配套齐全的10000余平方米生产车间;拥有完善的质量保证体系和健全质量控制制度;拥有多套高压液相色谱仪,气相色谱仪和紫外分光光度计等高效高灵敏度的分析仪器,对产品进行有效的分析和监控。公司已通过ISO9001质量体系认证。
公司拥有进出口自主权,公司生产的肉桂系列产品:肉桂酸、肉桂醛、肉桂醇、维生素E、维生素B1以及生物化工、医药中间体等产品畅销全国各地并出口到欧美及东南亚20多个国家和地区,深受客户好评。
公司投入巨资和人力用于研发适应市场需求的精细化工,生物化工,医药中间体等新产品,新工艺。高水平的研发团队,一流的实验设备,良好的科研氛围保证了研发实力,目前生产的产品,全部由本公司自行研究开发,达到了国内先进水平,部分产品达到国际先进水平。
公司坚持"以人为本,科技领先,远城服务,诚信永远"的服务宗旨。以质量求生存;以服务创效益;以法纪立信。
其他相关产品
[供]左旋肉碱?[供]左旋肉碱酒石酸盐? [供]生物素?[供]维生素B2(核黄素)?[供]L-精氨酸盐酸盐?[供]L-精氨酸盐酸盐?[供]维生素C?[供]维生素B1(盐酸硫胺)?[供]L-谷氨酰胺?[供]D-泛醇? [供]甘氨酸?[供]维A酸(维甲酸)?[供]烟酰胺?[供]植物甾醇(β-谷甾醇)?[供]羟丙基-β-环糊精?[供]维生素B6?[供]合成维生素E油?[供]β-胡萝卜素?[供]叶酸?[供]核黄素磷酸钠?[供]肌醇?[供]维生素B1?[供]L-谷氨酸
生产厂家,价格,原料