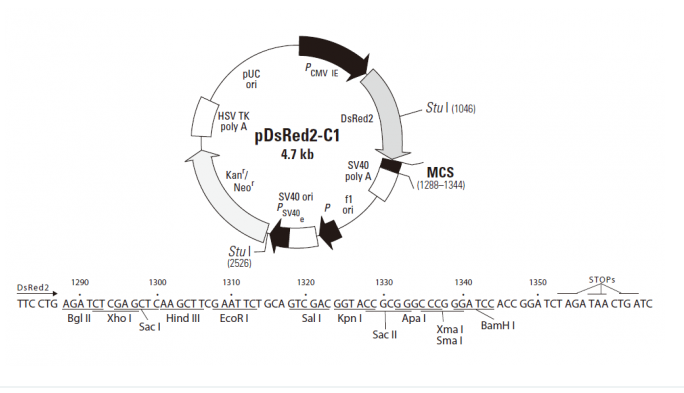
pDsRed2-C1 encodes DsRed2, a DsRed variant that has been engineered for faster maturation and lower non-specific aggregation. Derived from the Discosoma sp. red fluorescent protein (drFP583; 1), DsRed2, like its progenitor DsRed1, contains a series of silent base-pair changes that correspond to human codon-usage preferences for high expression in mammalian cells (2). In addition to these changes, DsRed2 contains six amino acid substitutions: V105A, I161T, and S197A, which result in the more rapid appearance of red fluorescence in transfected cell lines; and R2A, K5E, and K9T, which prevent the protein from aggregating. (DsRed2 may, however, form the same tetrameric structure as DsRed1 [3].) In mammalian cell cultures when DsRed2 is expressed constitutively, red-emitting cells can be detected by fluorescence microscopy within 24 hours of transfection. Large insoluble aggregates of protein, often observed in bacterial and mammalian cell systems expressing DsRed1, are dramatically reduced in organisms expressing DsRed2. The faster-maturing, more soluble red fluorescent protein is also well tolerated by host cells; mammalian cell cultures transfected with DsRed2 show no obvious signs of reduced viabilityin those cell lines tested, cells expressing DsRed2 display the same morphology (e.g., adherence, light-refraction) and growth characteristics as non-transfected controls.
The multiple cloning site (MCS) in pDsRed2-C1 is positioned between the DsRed2 coding sequence and the SV40 polyadenylation signal (SV40 poly A). Genes cloned into the MCS will be expressed as fusions to the C-terminus of DsRed2 if they are in the same reading frame as DsRed2 and there are no intervening stop codons. A Kozak consensus translation initiation site upstream of DsRed2 increases the translation efficiency in eukaryotic cells (4). SV40 poly A signals downstream of the MCS direct proper processing of the 3' end of mRNA transcripts. The vector backbone also contains an SV40 origin for replication in mammalian cells expressing the SV40 T-antigen, a pUC origin of replication for propagation in E. coli, and an f1 origin for single-stranded DNA production. A neomycin resistance cassette (Neor), consisting of the SV40 early promoter, the neomycin/kanamycin resistance gene of Tn5, and polyadenylation signals from the Herpes simplex virus thymidine kinase (HSV TK) gene, allows stably transfected eukaryotic cells to be selected using G418. A bacterial promoter upstream of this cassette expresses kanamycin resistance in E. coli.
载体应用
pDsRed2-C1 can be used to construct fusions to the C-terminus of DsRed2. If a fusion construct retains the fluorescent properties of the native DsRed2 protein, its expression can be monitored by flow cytometry and its localization in vivo can be determined by fluorescence microscopy. The target gene should be cloned into pDsRed2-C1 so that it is in frame with the DsRed2 coding sequences, with no intervening in-frame stop codons. The recombinant DsRed2 vector can be transfected into mammalian cells using any standard transfection method. If required, stable transformants can be selected using G418 (5). pDsRed2-C1 can also be used as a cotransfection marker; the unmodified vector will express DsRed2.