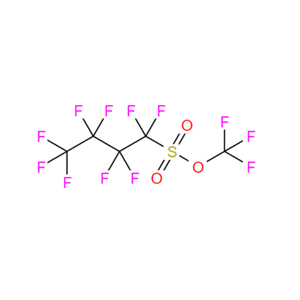
The triffuoromethoxy group (CF3O) is of particular
interest in pharmaceutical chemistry due to its unique
properties, such as moderate electronegativity (Hammett
constants σp = 0.35, σm = 0.38) and high lipophilicity (Hansch
parameter π = 1.04) effects.1 The successful development of
CF3O-containing pharmaceuticals, including Delamanid, Riluzole,
Sonidegib, and Pretomanid, demonstrates the high value
of the CF3O group, which has driven the chemical community
to devote signiffcant research efforts to developing triffuoromethoxylation
reagents and triffuoromethoxylation methods.2Many types of triffuoromethoxylation reagents have been
developed,3 such as [M+ CF3O−],4 SO2−OCF3,5 N−OCF3,6and C(O)−OCF3 types.7 The SO2−OCF3 reagents can release
CF3O− anions in the presence of a nucleophile, such as
ffuoride anions, which can readily attack the SO2 moiety to
cleave the SO2−OCF3 bond. The commonly used SO2−OCF3reagents include ArSO2−OCF3,5d−k CF3SO2−OCF3,5a−c and nC4F9SO2OCF3 (TFNf).5l ArSO2−OCF3 has served as a
versatile reagent for a wide variety of triffuoromethoxylation
reactions.5d−k CF3SO2−OCF3 has a low boiling point (19 °C)8and is highly volatile, which may limit its applications. In sharp
contrast, nC4F9SO2OCF3, developed as a triffuoromethoxylation
reagent by Hammond, Umemoto, and co-workers
recently,5l has a higher boiling point (87−89 °C) and thus is
more convenient for handling. The great synthetic potential of
TFNf, demonstrated with the regio- and stereoselectivity, and
wide functional group compatibility in triffuoromethoxylation
of alkynes,5l may stimulate research efforts to develop costeffective
methods for its preparation.
There have been three reports for the synthesis of nC4F9SO2OCF3. The ffrst report dates back to 1981, when
DesMarteau and Johri developed a two-step procedure starting
from nC4F9SO3H (Scheme 1, eq 1).9 This process requires the
use of a hazardous reagent, ClF, and hazardous Cl2 would also
be produced as a side product. Furthermore, the product of the
ffrst step, nC4F9SO3Cl, is quite unstable and would easily
decompose at room temperature. The second report
necessitates the use of a stoichiometric amount of a silver
salt, Ag2CO3, and it is quite difffcult to isolate nC4F9SO2OCF3from the reaction solvent, benzene, due to their similar boiling
points (eq 2).10 The latest method, described by Hammond,
Umemoto, and co-workers recently,5l starts from Umemoto’s
reagent11 to obtain nC4F9SO2OCF3 via anion exchanges and
thermolysis (eq 3). This method features an easy workup
procedure, just ffltration for the ffrst two steps and distillation
for the last step. However, the expensive Umemoto’s reagent is
required to be used as a starting material, which may restrict
the wide applications of nC4F9SO2OCF3.
Triffic anhydride (Tf2O) is an abundant and inexpensive
industrial raw material. In 2021, Ritter and co-workers
developed the synthesis of a S-CF3 thianthrenium salt by
using Tf2O as a CF3 source.12 The thianthrenium salt can act
as an efffcient electrophilic triffuoromethylation reagent. Based
on our previous studies on the electrophilicity of CF3-
containing organic salts,13 we speculated that S-CF3 thianthrenium
salts may undergo anion exchanges and thermolysis
to provide nC4F9SO2OCF3 (eq 4). In this process, all reagents
and starting materials are widely available, and no tedious
puriffcation procedure is required in any step. After isolatingReceived: December 16, 2022Published: February 10, 2023pubs.acs.org/joc Note© 2023 American Chemical Society 3346https://doi.org/10.1021/acs.joc.2c03018J. Org. Chem. 2023, 88, 3346−3352Downloaded via SH
AN
G
H
AI INST OF O
R
G
ANIC C
H
E
MISTR
Y on June 19, 2025 at 09:05:58 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.the ffnal nC4F9SO2OCF3 by distillation, thianthrene 1 can be
recycled simply by ffltration and washing with petroleum ether.
Ritter and co-workers used thianthrene 1a to synthesize
thianthrenium salt 2a.12 Besides 1a, we also examined other
thianthrenes containing electron-withdrawing groups (Table
1), since these groups may increase the electrophilicity of
thianthrenium salts to facilitate the ffnal thermolysis step.
However, electron-withdrawing groups would decrease the
efffciency of S-triffuoromethylation. The bi-CF3-substituted
thianthrenium salt was obtained in a low yield (2c), and theScheme 1. Synthesis of nC4F9SO2OCF3Table 1. Conversion of Thianthrenes into S-CF3 Thianthrenium SaltsaaIsolated yields are shown.Table 2. Two-Step Anion ExchangesaaIsolated yields are shown.The Journal of Organic Chemistry pubs.acs.org/joc Notehttps://doi.org/10.1021/acs.joc.2c03018J. Org. Chem. 2023, 88, 3346−33523347tetra-F-substituted substrate cannot be triffuoromethylated at
all (2d). Fortunately, the S-triffuoromethylation of di-Fsubstituted
thianthrene occurred smoothly (2b).
The direct anion exchange of TfO− with NfO− cannot occur
well because the physicochemical properties of both anions are
quite similar. Therefore, a two-step anion exchange was carried
out to afford nonaffuorobutanesulfonate (nonaffate) salts 4(Table 2). Both steps proceeded smoothly, and high yields
were obtained for each step. The products of each step can be
easily isolated by phase separation.
With the nonaffate salts 4 in hand, we then investigate the
ffnal thermolysis reactions (Table 3). Salt 4a can be converted
to give the desired product only in 43% 19F NMR yield. The
presence of electron-withdrawing groups can indeed facilitate
the thermolysis reactions (4b−4c). The reaction conditions of
thermolysis of 4b were screened (please see the Supporting
Information for details), and the highest yield obtained (82%)
is shown in entry 2. Although a high yield was obtained in the
case of 4c, the use of 4c may suffer from a low yield of the ffrst
step (2c, Table 1). The thermolysis of 4b gave a lower yield
compared with the case of 4c, but each step starting from
thianthrene 1b can take place smoothly. Therefore, 4b may be
considered as a good precursor of nC4F9SO2OCF3.
After the optimal conditions of the thermolysis of 4b was
identiffed, a gram scale reaction was performed (Scheme 2). nC4F9SO2OCF3 was isolated in 58% yield for the thermolysis
step (5.89 g), and the corresponding overall yield was
calculated to be 48%. Thianthrene 1b would be regenerated
from 4b via the thermolysis. After the isolation of nC4F9SO2OCF3 by distillation, thianthrene 1b was isolated
simply by ffltration and washing with petroleum ether (3.9 g,
56%). It is coincidental that the yield of the recovered 1b is
close to the isolated yield of nC4F9SO2OCF3.
In summary, we have described the development of an
efffcient route to a versatile triffuoromethoxylation reagent, nC4F9SO2OCF3. The abundant and inexpensive industrial raw
material, Tf2O, was used as a triffuoromethyl source, only
phase separation or distillation is needed for puriffcation, the
starting thianthrene can be recycled simply by ffltration and
washing, and the synthetic process can be easily scaled up.
These attracting features may widen the synthetic applications
of reagent nC4F9SO2OCF3.■ EXPERIMENTAL SECTION1. General Information. The 1H, 13C, and 19F NMR spectra were
recorded on 400 MHz NMR
![N-[(九氟代丁基)磺N-[(九氟代丁基)磺酰基]-1,1,2,2,3,3,4,4,4-九氟代-1-丁烷磺酰胺酰基]-1,1,2,2,3,3,4,4,4-九氟代-1-丁烷磺酰胺](https://img.chemicalbook.com/SupplyImg/2023-12-11/Large/202312111215052534336.png)