甲泼尼龙琥珀酸氢EP杂质F; 2376134-25-5 新品
Methylprednisolone Hydrogen Succinate EP Impurity F
2376134-25-5
2376134-25-5
询价
10mg
起订
50mg
起订
100mg
起订
湖北 更新日期:2026-03-03
产品详情:
- 中文名称:
- 甲泼尼龙琥珀酸氢EP杂质F
- 英文名称:
- Methylprednisolone Hydrogen Succinate EP Impurity F
- CAS号:
- 2376134-25-5
- 品牌:
- MOLCOO
- 产地:
- 武汉
- 保存条件:
- -20°C
- 纯度规格:
- 95% +HPLC
- 产品类别:
- 杂质对照品
- 货号:
- M038022
- 是否进口:
- 否
- 用途:
- 药物研发
- 产品规格:
- mg
- 分子式::
- C26H34O8
- 分子量::
- 474.54
公司简介
摩科MOLCOO拥有专业的药物肽合成团队,可根据客户提供的多肽序列进行药物肽定制合成,也可为客户提供多肽药物研发中产生的各类降解杂质、工艺杂质现货。我们提供常规结构确诊谱图资料如质谱、液相、紫外光谱图,还可根据客户需求提供氨基酸组成分析、氨基酸序列测定等资料。根据客户项目要求纯度范围一般为90%-99%。
其他业务:药物杂质对照品、杂质及新分子定制合成、未知杂质制备分离、新药中间体工艺开发等。
| 成立日期 | (8年) |
| 注册资本 | 1000万人民币 |
| 员工人数 | 50-100人 |
| 年营业额 | ¥ 1000万-5000万 |
| 经营模式 | 工厂,定制,服务 |
| 主营行业 | 生化试剂,核苷,核苷酸,寡核苷酸,蛋白组学,有机合成试剂,氨基酸及其衍生物 |
甲泼尼龙琥珀酸氢EP杂质F相关厂家报价
-

- 甲泼尼龙琥珀酸酯杂质2;11-脱氢甲泼尼龙琥珀酸酯
- 廊坊市泽康医药科技有限公司 VIP
- 2026-01-23
- 询价
-
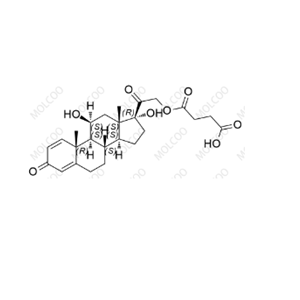
- 甲泼尼龙琥珀酸氢EP杂质 E
- 深圳摩科生化科技有限公司 VIP
- 2026-03-06
- 询价
-
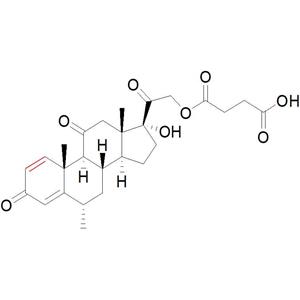
- 甲泼尼龙琥珀酸酯杂质2;11-脱氢甲泼尼龙琥珀酸酯
- 廊坊市恒福源医药科技有限公司 VIP
- 2026-01-13
- 询价
-
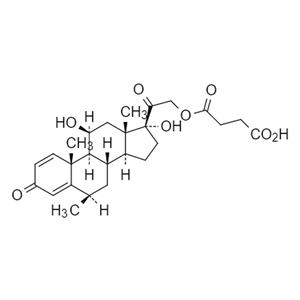
- 6β琥珀酸甲泼尼龙
- 天津那瑞医药科技有限公司 VIP
- 2026-02-12
- 询价
-

- 甲泼尼龙琥珀酸氢EP杂质F 2376134-25-5
- 深圳市恒丰万达医药科技有限公司 VIP
- 2026-02-09
- 询价





