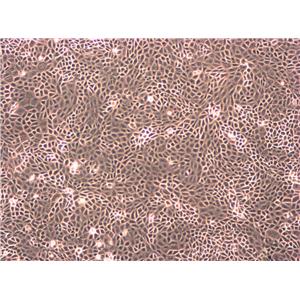
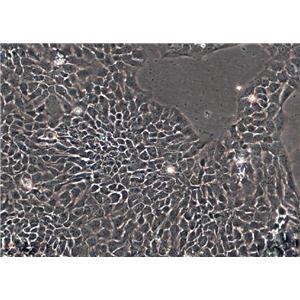
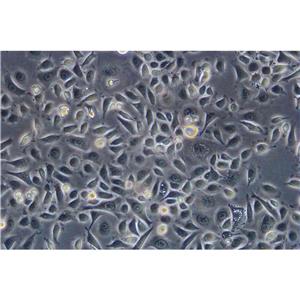
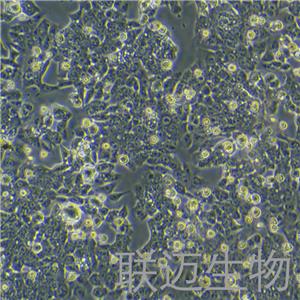
"HCT 116人结肠癌复苏细胞保种中心|带STR证书
传代比例:1:2-1:4(首次传代建议1:2)
生长特性:贴壁生长
细胞传代培养实验:体外培养的原代细胞或细胞株要在体外持续地培养就必须传代,以便获得稳定的细胞株或得到大量的同种细胞,并维持细胞种的延续。培养的细胞形成单层汇合以后,由于密度过大生存空间不足而引起营养枯竭,将培养的细胞分散,从容器中取出,以1:2或1:3以上的比率转移到另外的容器中进行培养,即为传代培养;细胞“一代”指从细胞接种到分离再培养的一段期间,与细胞世代或倍增不同。在一代中,细胞培增3~6次。细胞传代后,一般经过三个阶段:游离期、指数增生期和停止期。常用细胞分裂指数表示细胞增殖的旺盛程度,即细胞群的分裂相数/100个细胞。一般细胞分裂指数介于0.2%~0.5%,肿瘤细胞可达3~5%;细胞接种2~3天分裂增殖旺盛,是活力ZuiHAO时期,称指数增生期(对数生长期),适宜进行各种试验。实验步骤:1.将长成的培养细胞从二氧化碳培养箱中取出,在超净工作台中倒掉瓶内的培养,加入少许消化。(以面盖住细胞为宜),静置5~10分钟。2.在倒置镜下观察被消化的细胞,如果细胞变圆,相互之间不再连接成片,这时应立即在超净台中将消化倒掉,加入3~5ml新鲜培养,吹打,制成细胞悬。3.将细胞悬吸出2ml左右,加到另一个培养瓶中并向每个瓶中分别加3ml左右培养,盖HAO瓶塞,送回二氧化碳培养箱中,继续进行培养。一般情况,传代后的细胞在2小时左右就能附着在培养瓶壁上,2~4天就可在瓶内形成单层,需要再次进行传代。
换液周期:每周2-3次
NSC-34 Cells;背景说明:神经元;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:CMK细胞、FRhK-4细胞、SKRC 39细胞
H-2087 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:4传代;每周换液2次。;生长特性:悬浮生长,有少数细胞疏松贴壁;形态特性:上皮样;相关产品有:PL-12细胞、NIH/3T3细胞、MDA-MB-453细胞
TEC Cells;背景说明:胸腺;上皮 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:BNL.CL2细胞、SK Mel 2细胞、LM3细胞
HCT 116人结肠癌复苏细胞保种中心|带STR证书
背景信息:是一种人结肠癌细胞系,是由M·Brattain等人于1979年从患结肠癌的男性病人中分离的三株恶性细胞中的一株。HCT 116细胞在半固体琼脂糖培养基中形成克隆;HCT 116细胞在无胸腺裸鼠有致瘤性,形成肿瘤结节。
细胞系的应用:1)免疫组化研究2)RNA干扰研究3)药物作用研究4)慢病毒转染研究等其它应用。细胞系通常用于实验研究,如增殖、迁移、侵袭等。细胞系在多个领域的研究中被广泛应用,包括基础医学、临床试验、药物筛选和分子生物学研究。这些研究不仅在中国,也在日本、美国和欧洲等多个国家和地区进行。
产品包装:复苏发货:T25培养瓶(一瓶)或冻存发货:1ml冻存管(两支)
来源说明:细胞主要来源ATCC、ECACC、DSMZ、RIKEN等细胞库
RPMI no. 8226 Cells;背景说明:来源于一位61岁的男性浆细胞瘤患者;可产生免疫球蛋白轻链,未检测到重链。;传代方法:按1:2传代,5-6小时可以看到细胞分裂;生长特性:悬浮生长;形态特性:淋巴母细胞样;相关产品有:BHT-101细胞、G292细胞、Rat Basophilic Leukemia-1细胞
MFD-1 Cells;背景说明:详见相关文献介绍;传代方法:维持细胞浓度在3×104-5×105 cells/ml;2-3天换液1次。;生长特性:悬浮生长;形态特性:淋巴母细胞样 ;相关产品有:LAPC-4细胞、JHH-7细胞、MC3T3-E1 Subclone 24细胞
SK-NMC Cells;背景说明:这株细胞与HTB-11都是神经源的。1971年9月分离得到SK-N-MC后,发现它有中性多巴胺-β-羟化酶活性,也有细胞内儿茶胺,用甲醛可以诱导出荧光。;传代方法:1:6-1:12传代,每周2-3次换液。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Swiss 3T6细胞、PAI细胞、MAEC细胞
SUPB-15 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代。3天内可长满。;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:CCD-841-CoN细胞、HCT-8细胞、NCI-H446细胞
HCT 116人结肠癌复苏细胞保种中心|带STR证书
物种来源:人源、鼠源等其它物种来源
形态特性:上皮细胞样
其实绝大部分细胞消化的时候是只要用胰酶润洗一遍即可,吸去胰酶后,残留的那些无法计算体积的附着在细胞表面的微量胰酶在37度一般不到2min足够消化细胞(绝大部分1min不到)。对于这些细胞原则上不要用胰酶孵育细胞,连续这样传代,对细胞伤害很大。简单的程序是PBS润洗吸去,胰酶润洗吸去,然后37度消化;什么算是消化HAO了呢?不是细胞全部成间隔分布很离散的单个圆形才算消化HAO了,一般你肉眼观察贴壁细胞层,只要能移动了,多半呈沙壮移动,其实已经可以了,很多人喜欢把细胞消化或者吹打成完全分离细胞,这是没有必要的。一般能移动了,说明细胞与培养基质材料的附着已经消失了,细胞之间的附着也已经消失了,细胞已经独立分布了(虽然没有呈现很广的离散分布)。这个时候应该停止消化,不要等到看到镜下所有细胞都分离得非常HAO,间隙很大,才停止。细胞就是完全成单个细胞悬,之后在贴壁的过程中仍然会聚集,这个是贴壁培养的细胞,尤其是肿瘤细胞的一个性,无论死活的细胞都是如此,你可以尝试,准备100%的单个细胞悬,贴壁后观察细胞,仍然是几个几个细胞聚集在一起。一些悬浮培养细胞也是如此,容易聚集,不要去尝试过几个小时就拿出来吹打成单细胞悬(不要笑,这个是初养悬浮细胞的人常犯的错误,以为悬浮培养就是一个一个分开)。细胞只要能从基质上脱离下来,这个时候即使是成片的(比如Calu-3细胞),吹打不超过20次后(一般10次即可),成小规模聚集(10个细胞左右),是正常的,不要试图再去延长消化时间,或者像有的同学那样吹打1h,等待单细胞悬出现。
BrCL15 Cells;背景说明:MDA-MB-435S是一种纺锤形的细胞,1976年由其亲本(435)中筛选得到。435是从31岁的转移性乳腺导管腺癌女性患者胸水中分离得到。当用荧光染料对微管蛋白进行染色时亲本细胞显现散布特征(II型)。最近通过cDNA阵列研究表明,亲本(MDA-MB-435)可归入黑素瘤起源。;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:纺锤形;相关产品有:Hep II细胞、Cates 1B细胞、P388-D1细胞
Lewis lung carcinoma line 1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MDA157细胞、IPEC-1细胞、SNU119细胞
Duke University 145 Cells;背景说明:DU 145 是从一位有3年淋巴细胞白血病史的前列腺癌患者的脑部转移灶中建立的。该细胞系未检测到激素敏感性,酸性酶阳性,单个的细胞可在软琼脂中形成集落。对此细胞和原始肿瘤的亚显微结构分析可见微绒毛、微丝、细胞桥粒、线粒体、发达的高尔基体和异质溶酶体。该细胞不表达前列腺抗原。;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:上皮细胞样;相关产品有:M619细胞、D-283 Med细胞、Japanese Tissue Culture-39细胞
SVHUC Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明书;相关产品有:H-2052细胞、Factor Dependent Cell-Paterson 1细胞、NCI-H716细胞
Namalwa Cells;背景说明:这株细胞有EB病毒基因组。;传代方法:1:2传代。3天内可长满。;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:A 253细胞、CV-1 in Origin Simian-7细胞、Mc Ardle 7777细胞
CHO-K1 Cells;背景说明:1957年,PuckTT从成年中国仓鼠卵巢的活检组织建立了CHO细胞,CHO-K1是CHO的一个亚克隆。CHO-K1的生长需要脯酸。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:V 79细胞、MCF 7细胞、NCI-HUT-69细胞
U-87MG ATCC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:BJ [Human fibroblast]细胞、SW 1222细胞、HT-1376细胞
KYSE 270 Cells;背景说明:详见相关文献介绍;传代方法:1:5传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:HOP92细胞、Hs739T细胞、G-361细胞
OCI-AML5 Cells;背景说明:急性髓系白血病细胞;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:JURKAT E-6.1细胞、HCe-8693细胞、GM07404A细胞
A9 (Hamprecht) Cells;背景说明:皮下结缔组织;自发永生;雄性;C3H/An;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:HLF细胞、CFSC-2G细胞、HEK293S细胞
EU-3 Cells;背景说明:B淋巴细胞白血病;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:PANC0203细胞、Vero 76细胞、DHL-5细胞
NIE 115 Cells;背景说明:神经母细胞瘤;雄性;A/J;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:B16-F10-BL6细胞、Bac1 2F5细胞、NCIH1395细胞
Hs 636 T Cells;背景说明:宫颈鳞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:SNG-M细胞、Hs.27细胞、A-101D细胞
OCIAML3 Cells;背景说明:急性髓系白血病;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:BetaTC6细胞、GM2132细胞、HS-5细胞
Panc-3_27 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:WiDr细胞、SUIT 2细胞、PC3M-2B4细胞
Hs 675.T Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代,每周2-3次。;生长特性:贴壁生长;形态特性:成纤维细胞;相关产品有:PanC1细胞、KYSE-270细胞、NHEK细胞
SW480E Cells;背景说明:SW480源自一位51岁白人男性患者的原位直肠腺癌,而SW620源自同一病人一年后的淋巴结转移灶。该细胞CSAp和直肠抗原3阴性;角蛋白阳性;p53基因第273位密码子的G→A突变引起Arg→His替代,309位密码子的C→T突变导致Pro→Ser替代;细胞p53蛋白表达水平升高;癌基因c-myc、K-ras、H-ras、N-ras、myb、sis和fos的表达呈阳性;未检测到癌基因N-myc的表达;不表达Matrilysin(一种与肿瘤侵袭相关的金属蛋白酶)。;传代方法:1:2传代,1-2天换液一次;生长特性:贴壁生长;形态特性:上皮样;相关产品有:MDA-361细胞、NCI-H650细胞、SU-DH-L5细胞
HCT 116人结肠癌复苏细胞保种中心|带STR证书
Abcam A-549 CHD7 KO Cells(提供STR鉴定图谱)
Abcam THP-1 ADGRG1 KO Cells(提供STR鉴定图谱)
BayGenomics ES cell line CSA051 Cells(提供STR鉴定图谱)
BayGenomics ES cell line RRS338 Cells(提供STR鉴定图谱)
BayGenomics ES cell line YTA554 Cells(提供STR鉴定图谱)
chHES-145 Cells(提供STR鉴定图谱)
DA01956 Cells(提供STR鉴定图谱)
DA05407 Cells(提供STR鉴定图谱)
GeneBLAzer D5-CRE-bla CHO-K1 Cells(提供STR鉴定图谱)
SU-DHL-6 Cells;背景说明:详见相关文献介绍;传代方法:1:3—1:6传代,3—4天换液1次;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:SL-1细胞、H2227细胞、B-CPAP细胞
CORL23 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:DoTc2细胞、Madin Darby Bovine Kidney细胞、RF/6A细胞
RCS Cells;背景说明:软骨肉瘤;SD;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:A375细胞、GM17219细胞、PANC3.27细胞
Dysplastic Oral Keratinocyte Cells;背景说明:口腔异常增生;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:ACC2细胞、SK-RC-42细胞、SKMEL1细胞
MDA-kb2 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明书;相关产品有:COLO-699细胞、ketr 3细胞、P3/NS1/1-Ag4-1细胞
Anip[973] Cells;背景说明:肺腺癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:B16BL6细胞、WTRL1细胞、293-EBNA细胞
1D2 [Mouse hybridoma against human RPSA] Cells(提供STR鉴定图谱)
RBL 2H3 Cells;背景说明:详见相关文献介绍;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:成纤维细胞;相关产品有:NCIH2023细胞、UPCI-SCC-90细胞、C4-2细胞
HEEpiC Cells;背景说明:食管;上皮 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Stanford University-Diffuse Histiocytic Lymphoma-6细胞、BE(2)-C细胞、JCA1细胞
UCH1 Cells;背景说明:骶骨脊索瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:HBZY1细胞、H-650细胞、P3J HR1-K细胞
CCD 1112SK Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:OVCAR-432细胞、OV1-P细胞、H-1650细胞
SK-BR3 Cells;背景说明:这株细胞源自胸水。没有病毒颗粒。亚显微结构特征包括微丝和桥粒,肝糖原颗粒,大溶酶体,成束的细胞质纤丝。SK-BR-3细胞株过表达HER2/c-erb-2基因产物。;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:上皮样;相关产品有:MKN-74细胞、DMS-114细胞、Tohoku Hospital Pediatrics-1细胞
KRC/Y Cells;背景说明:肾透明细胞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:VMM5细胞、GT1-1细胞、P31/FUJ细胞
KRC/Y Cells;背景说明:肾透明细胞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:VMM5细胞、GT1-1细胞、P31/FUJ细胞
HT 1197 Cells;背景说明:详见相关文献介绍;传代方法:1:5-1:10传代,2天换液1次。;生长特性:贴壁生长;形态特性:详见产品说明书;相关产品有:RFL 6细胞、HEK293-FT细胞、IALM细胞
Grunt Fin Cells(提供STR鉴定图谱)
HAP1 POT1 (-) 1 Cells(提供STR鉴定图谱)
H1648 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:HFSF细胞、Wills Eye Research Institute-Retinoblastoma-1细胞、HBE细胞
HepG2 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:Centre Antoine Lacassagne-148细胞、Primary Liver Carcinoma/Poliomyelitis Research Foundation/5细胞、JKT1细胞
MSC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:CALU1细胞、GT38细胞、ARPE-19细胞
HN4 Cells;背景说明:喉鳞癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:LA4细胞、MDA361细胞、H-1618细胞
HBEpiC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:RWPE-2细胞、SKO3细胞、GM03571细胞
LN382 Cells;背景说明:胶质瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:526mel细胞、PL5细胞、GC1-SPG细胞
SKGIIIA Cells;背景说明:详见相关文献介绍;传代方法:2x10^4 cells/ml;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:Strain L-929细胞、MDAMB415细胞、H1672细胞
Centre Antoine Lacassagne-12T Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MDA MB 134VI细胞、HOS细胞、Bronchial Epithelium transformed with Ad12-SV40 2B细胞
hTERT NF1 ipNF08.1.5 Cells(提供STR鉴定图谱)
KTCTL-140 Cells(提供STR鉴定图谱)
MRC-5 SV1 TG1 Cells(提供STR鉴定图谱)
NZP-46 Cells(提供STR鉴定图谱)
RKC1 Cells(提供STR鉴定图谱)
Ubigene HCT 116 TNFRSF10B KO Cells(提供STR鉴定图谱)
WAe014-A-1 Cells(提供STR鉴定图谱)
HG02010 Cells(提供STR鉴定图谱)
SW 1783 Cells;背景说明:间变性星形细胞瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:BEL7404细胞、P-388细胞、LTPA细胞
OCI-LY-18 Cells;背景说明:弥漫大B细胞淋巴瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:ROS17/2.8细胞、MCA38细胞、HCC-9724细胞
MAVER Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:5传代;2-3天换液1次。;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:HuH 6细胞、FL-83B细胞、P3.NS-1/1.Ag4.1细胞
MeSoTheliOma-211H Cells;背景说明:MSTO-211H细胞株是1985年从一位肺二相间皮瘤患者的胸水中建株的。这个病人接受过多种药物联合前期化疗。MSTO-211H细胞具有高亲和力的EGF结合位点,并表达神经元特异性烯醇酶(NSE)及人绒毛膜促性腺激素(HCG)的α与β亚基。未检测到左旋多巴胺脱羧酶(DDC),邦巴辛与神经tensin。细胞过表达c-myc原癌基因,并没有观察到基因重排或扩增。V-src,v-abl,v-erbB,c-raf1,Ha-ras,Ki-ras,和N-ras的表达呈阳性。未检测到N-m;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:成纤维细胞样;相关产品有:BIU-87细胞、Instituto Biologico-Rim Suino-2细胞、SU-DHL-1细胞
SW-1463 Cells;背景说明:详见相关文献介绍;传代方法:1:3—1:8传代,每周换液1-2次;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:HO1-N-1细胞、MCF.7细胞、BEAS2B细胞
SW-1463 Cells;背景说明:详见相关文献介绍;传代方法:1:3—1:8传代,每周换液1-2次;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:HO1-N-1细胞、MCF.7细胞、BEAS2B细胞
746T Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MNNG-HOS (Cl#5)细胞、PC3M-2B4细胞、VM-CUB1细胞
C518 Cells;背景说明:膝关节退变软骨 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:MES-SA/Dx-5细胞、SV40 MES 13细胞、HLE-SRA-01/04细胞
H211 Cells;背景说明:详见相关文献介绍;传代方法:3-4天换液1次。;生长特性:悬浮生长;形态特性:详见产品说明书;相关产品有:UCLA-SO-M21细胞、HCa/16A3-F细胞、HGE细胞
293 H Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:RGC5细胞、NCI-H2291细胞、H-125细胞
SW13 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:NCIH1436细胞、WERI-Rb1细胞、hTERT-HME-1细胞
SW1116 Cells;背景说明:CSAp阴性(CSAp-)。 结肠抗原3,阴性。 角蛋白免疫过氧化物酶染色阳性。 癌基因c-myc, K-ras, H-ras, myb, sis 和fos的表达呈阳性。 未检测到癌基因N-myc和N-ras的表达。 表达肿瘤特异的核基质蛋白CC-4,CC-5和CC-6。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:KYSE0520细胞、HT-1197细胞、NCI-H719细胞
NS1-1 Ag4.1 Cells;背景说明:这是P3X63Ag8(ATCCTIB-9)的一个不分泌克隆。Kappa链合成了但不分泌。能抗0.1mM8-氮杂鸟嘌呤但不能在HAT培养基中生长。据报道它是由于缺失了3-酮类固醇还原酶活性的胆固醇营养缺陷型。检测表明肢骨发育畸形病毒(鼠痘)阴性。;传代方法:1:2传代,3天内可长满。;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:H-1238细胞、PANC1005细胞、EB3 [Human Burkitt lymphoma]细胞
PLA801D Cells;背景说明:这是一株高转移肺癌。;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:上皮样;相关产品有:P388-D1细胞、LU-65M细胞、LTEP-a-2细胞
RPE1 Cells;背景说明:视网膜色素上皮;hTERT永生;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:WM 266-4细胞、H-2195细胞、CCD-112 CoN细胞
SVts7-1 Cells(提供STR鉴定图谱)
RWPE1 Cells;背景说明:肿瘤抑制基因: p53 + [PubMed: 9214605] pRB + [PubMed: 9214605] 一位正常男性前列腺组织切片的周围区域的上皮细胞用单拷贝的人乳头瘤病毒的18(HPV-18)进行转化,建立了RWPE-1 (ATCC CRL-11609) 细胞株 [PubMed: 9214605]. 在三维Matrigel培养时,在雄激素作用下,RWPE-1细胞形成腺胞并向培养基中分泌PSA。[PubMed: 11170142]. 当与Matrigel或基质细胞混合注射雄性裸鼠时,RWPE-1细胞也能形成腺胞[PubMed: 11304724] 并产生PSA。 来源于RWPE-1的细胞再用Kirstin鼠类肿瘤病毒转染Ki-ras基因,建立了能成瘤的RWPE-2细胞株(ATCC CRL-11610) [PubMed: 9214605] 和 RWPE2-W99 (ATCC CRL-2853) 细胞株。 另外,用N--N-(MNU)处理RWPE-1,建立了一系列模拟前列腺癌进程中不同时期的成瘤细胞株。 它们是 WPE1-NA22 (ATCC CRL-2849), WPE1-NB14 (ATCC CRL-2850, WPE1-NB11 (ATCC CRL-2851) 和 WPE1-NB26 (ATCC CRL-2852) 细胞株。 据提供者报道,RWPE-1 细胞株(ATCC CRL-11609)经过检测,乙肝、丙肝、人免疫缺陷病毒都呈阴性。;传代方法:1:3传代,2-3天传一代。;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:Walker-256细胞、mIMCD3细胞、P3-X63-Ag8.653细胞
NCI.H226 Cells;背景说明:1980年分离建立。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:HEC1-B细胞、5-8F细胞、NCI H508细胞
ME180 Cells;背景说明:这株细胞来源于一个细胞集落不规则没有显著角质化的侵染性鳞状细胞癌。 单层培养的细胞间可以观察到带状连接,也注意到有细胞质张力丝。 1970年发现支原体污染并去除。 肿瘤坏死因子(TNF)α抑制ME-180的生长。 这株细胞含有人乳头瘤病毒(HPV)DNA,与HPV-39的同源性高于HPV-18。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:PNT1-a细胞、L78细胞、Fetal Human Colon细胞
SK.OV.3 Cells;背景说明:SK-OV-3由G.Trempe和L.J.Old在1973年从卵巢肿瘤病人的腹水分离得到。 此细胞对肿瘤坏死因子和几种细胞毒性药物包括白喉毒素、顺铂和阿霉素均耐受。 在裸鼠中致瘤,且形成与卵巢原位癌一致的中度分化的腺癌。;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:上皮细胞样;相关产品有:HME1细胞、NUGC2细胞、Emory University-2细胞
NMC-G1 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:MB157细胞、HLCL9B10细胞、PanC1细胞
OCI-LY-3 Cells;背景说明:弥漫大B淋巴瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:MEC-1细胞、SKml2细胞、HIT细胞
Hi-5 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:HCC1438细胞、SNU-C2A细胞、As-PC1细胞
SCC7 Cells;背景说明:鳞状细胞癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Douglas Foster-1细胞、ASH3细胞、Balb/3T3-4-Cl31细胞
SU-DH-L5 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:PC 61细胞、RetroPack PT67细胞、EBTr细胞
Sc-1 Cells;背景说明:胚胎;自发永生;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:RS 4;11细胞、HOC1细胞、MFE 296细胞
MESSA Cells;背景说明:详见相关文献介绍;传代方法:1:6-1:8传代;每周2-3次。;生长特性:贴壁生长;形态特性:成纤维细胞样 ;相关产品有:M109细胞、4-1st细胞、HL-60 Clone 15细胞
DU 4475 Cells;背景说明:详见相关文献介绍;传代方法:每周换液2—3次;生长特性:悬浮,多细胞聚集;形态特性:上皮细胞样;相关产品有:WRL68细胞、TF-1细胞、MonoMac 6细胞
J111 Cells;背景说明:单核细胞白血病;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:CF Pac1细胞、MDA-MB-134-VI细胞、NBL-S细胞
MDAMB-231 Cells;背景说明:MDA-MB-231来自患有转移乳腺腺癌的51岁女病人的胸水。在裸鼠和ALS处理的BALB/c小鼠中,它能形成低分化腺癌(III级)。;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:上皮样;相关产品有:OVCA5细胞、Hs695T细胞、VAESBJ细胞
HCT 116人结肠癌复苏细胞保种中心|带STR证书
BayGenomics ES cell line RRG143 Cells(提供STR鉴定图谱)
BayGenomics ES cell line XG876 Cells(提供STR鉴定图谱)
Ep-C4 Cells(提供STR鉴定图谱)
MUC 10-22 Cells(提供STR鉴定图谱)
Ubigene 4T1 Fat1 KO Cells(提供STR鉴定图谱)
Maja Cells(提供STR鉴定图谱)
" "PubMed=2835152
Boyd D., Florent G., Kim P., Brattain M.G.
Determination of the levels of urokinase and its receptor in human colon carcinoma cell lines.
Cancer Res. 48:3112-3116(1988)
PubMed=3335022
Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R.
Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay.
Cancer Res. 48:589-601(1988)
PubMed=2041050; DOI=10.1093/jnci/83.11.757
Monks A., Scudiero D.A., Skehan P., Shoemaker R.H., Paull K.D., Vistica D.T., Hose C.D., Langley J., Cronise P., Vaigro-Wolff A., Gray-Goodrich M., Campbell H., Mayo J.G., Boyd M.R.
Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines.
J. Natl. Cancer Inst. 83:757-766(1991)
PubMed=7972006; DOI=10.1073/pnas.91.23.11045; PMCID=PMC45163
Okamoto A., Demetrick D.J., Spillare E.A., Hagiwara K., Hussain S.P., Bennett W.P., Forrester K., Gerwin B.I., Serrano M., Beach D.H., Harris C.C.
Mutations and altered expression of p16INK4 in human cancer.
Proc. Natl. Acad. Sci. U.S.A. 91:11045-11049(1994)
PubMed=7761852; DOI=10.1126/science.7761852
Markowitz S.D., Wang J., Myeroff L.L., Parsons R., Sun L.-Z., Lutterbaugh J.D., Fan R.S., Zborowska E., Kinzler K.W., Vogelstein B., Brattain M.G., Willson J.K.V.
Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability.
Science 268:1336-1338(1995)
PubMed=7824277
Eshleman J.R., Lang E.Z., Bowerfind G.K., Parsons R., Vogelstein B., Willson J.K.V., Veigl M.L., Sedwick W.D., Markowitz S.D.
Increased mutation rate at the hprt locus accompanies microsatellite instability in colon cancer.
Oncogene 10:33-37(1995)
PubMed=9000147
Cottu P.-H., Muzeau F., Estreicher A., Flejou J.-F., Iggo R.D., Thomas G., Hamelin R.
Inverse correlation between RER+ status and p53 mutation in colorectal cancer cell lines.
Oncogene 13:2727-2730(1996)
PubMed=9000572
Hoang J.-M., Cottu P.-H., Thuille B., Salmon R.J., Thomas G., Hamelin R.
BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines.
Cancer Res. 57:300-303(1997)
PubMed=9023415; DOI=10.1006/cimm.1996.1062
Seki N., Hoshino T., Kikuchi M., Hayashi A., Itoh K.
HLA-A locus-restricted and tumor-specific CTLs in tumor-infiltrating lymphocytes of patients with non-small cell lung cancer.
Cell. Immunol. 175:101-110(1997)
PubMed=9178645; DOI=10.1006/cimm.1997.1108
Nakao M., Sata M., Saitsu H., Yutani S., Kawamoto M., Kojiro M., Itoh K.
CD4+ hepatic cancer-specific cytotoxic T lymphocytes in patients with hepatocellular carcinoma.
Cell. Immunol. 177:176-181(1997)
PubMed=9294210; DOI=10.1073/pnas.94.19.10330; PMCID=PMC23362
Ilyas M., Tomlinson I.P.M., Rowan A.J., Pignatelli M., Bodmer W.F.
Beta-catenin mutations in cell lines established from human colorectal cancers.
Proc. Natl. Acad. Sci. U.S.A. 94:10330-10334(1997)
PubMed=9515795
Sparks A.B., Morin P.J., Vogelstein B., Kinzler K.W.
Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer.
Cancer Res. 58:1130-1134(1998)
PubMed=9715273; DOI=10.1038/sj.onc.1201986
Eshleman J.R., Casey G., Kochera M.E., Sedwick W.D., Swinler S.E., Veigl M.L., Willson J.K.V., Schwartz S., Markowitz S.D.
Chromosome number and structure both are markedly stable in RER colorectal cancers and are not destabilized by mutation of p53.
Oncogene 17:719-725(1998)
PubMed=10674020; DOI=10.1016/S0959-8049(99)00206-3
Ku J.-L., Yoon K.-A., Kim D.-Y., Park J.-G.
Mutations in hMSH6 alone are not sufficient to cause the microsatellite instability in colorectal cancer cell lines.
Eur. J. Cancer 35:1724-1729(1999)
PubMed=10612807; DOI=10.1002/(SICI)1098-2264(200002)27:2<183::aid-gcc10>3.0.CO;2-P; PMCID=PMC4721570
Ghadimi B.M., Sackett D.L., Difilippantonio M.J., Schrock E., Neumann T., Jauho A., Auer G., Ried T.
Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations.
Genes Chromosomes Cancer 27:183-190(2000)
PubMed=10700174; DOI=10.1038/73432
Ross D.T., Scherf U., Eisen M.B., Perou C.M., Rees C., Spellman P.T., Iyer V.R., Jeffrey S.S., van de Rijn M., Waltham M.C., Pergamenschikov A., Lee J.C.F., Lashkari D., Shalon D., Myers T.G., Weinstein J.N., Botstein D., Brown P.O.
Systematic variation in gene expression patterns in human cancer cell lines.
Nat. Genet. 24:227-235(2000)
PubMed=10700188; DOI=10.1038/73536
Gayther S.A., Batley S.J., Linger L., Bannister A.J., Thorpe K., Chin S.-F., Daigo Y., Russell P., Wilson A., Sowter H.M., Delhanty J.D.A., Ponder B.A.J., Kouzarides T., Caldas C.
Mutations truncating the EP300 acetylase in human cancers.
Nat. Genet. 24:300-303(2000)
PubMed=10737795; DOI=10.1073/pnas.97.7.3352; PMCID=PMC16243
Rowan A.J., Lamlum H., Ilyas M., Wheeler J.M.D., Straub J., Papadopoulou A., Bicknell D.C., Bodmer W.F., Tomlinson I.P.M.
APC mutations in sporadic colorectal tumors: a mutational 'hotspot' and interdependence of the 'two hits'.
Proc. Natl. Acad. Sci. U.S.A. 97:3352-3357(2000)
PubMed=11226274; DOI=10.1073/pnas.041603298; PMCID=PMC30173
Abdel-Rahman W.M., Katsura K., Rens W., Gorman P.A., Sheer D., Bicknell D.C., Bodmer W.F., Arends M.J., Wyllie A.H., Edwards P.A.W.
Spectral karyotyping suggests additional subsets of colorectal cancers characterized by pattern of chromosome rearrangement.
Proc. Natl. Acad. Sci. U.S.A. 98:2538-2543(2001)
PubMed=11314036; DOI=10.1038/sj.onc.1204211
Forgacs E., Wren J.D., Kamibayashi C., Kondo M., Xu X.L., Markowitz S.D., Tomlinson G.E., Muller C.Y., Gazdar A.F., Garner H.R., Minna J.D.
Searching for microsatellite mutations in coding regions in lung, breast, ovarian and colorectal cancers.
Oncogene 20:1005-1009(2001)
PubMed=11414198; DOI=10.1007/s004320000207
Lahm H., Andre S., Hoeflich A., Fischer J.R., Sordat B., Kaltner H., Wolf E., Gabius H.-J.
Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures.
J. Cancer Res. Clin. Oncol. 127:375-386(2001)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=11526487; DOI=10.1038/sj.onc.1204611
Gayet J., Zhou X.-P., Duval A., Rolland S., Hoang J.-M., Cottu P.-H., Hamelin R.
Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines.
Oncogene 20:5025-5032(2001)
PubMed=11687795; DOI=10.1038/ng754
Snijders A.M., Nowak N.J., Segraves R., Blackwood S., Brown N., Conroy J., Hamilton G., Hindle A.K., Huey B., Kimura K., Law S., Myambo K., Palmer J., Ylstra B., Yue J.P., Gray J.W., Jain A.N., Pinkel D., Albertson D.G.
Assembly of microarrays for genome-wide measurement of DNA copy number.
Nat. Genet. 29:263-264(2001)
PubMed=12068308; DOI=10.1038/nature00766
Davies H.R., Bignell G.R., Cox C., Stephens P.J., Edkins S., Clegg S., Teague J.W., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C.S., Shipley J.M., Hargrave D., Pritchard-Jones K., Maitland N.J., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A.M., Nicholson A., Ho J.W.C., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H.F., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A.
Mutations of the BRAF gene in human cancer.
Nature 417:949-954(2002)
PubMed=12584437; DOI=10.1159/000068544
Melcher R., Koehler S., Steinlein C., Schmid M., Mueller C.R., Luehrs H., Menzel T., Scheppach W., Moerk H., Scheurlen M., Koehrle J., Al-Taie O.
Spectral karyotype analysis of colon cancer cell lines of the tumor suppressor and mutator pathway.
Cytogenet. Genome Res. 98:22-28(2002)
PubMed=12615714
Hempen P.M., Zhang L., Bansal R.K., Iacobuzio-Donahue C.A., Murphy K.M., Maitra A., Vogelstein B., Whitehead R.H., Markowitz S.D., Willson J.K.V., Yeo C.J., Hruban R.H., Kern S.E.
Evidence of selection for clones having genetic inactivation of the activin A type II receptor (ACVR2) gene in gastrointestinal cancers.
Cancer Res. 63:994-999(2003)
PubMed=12671075; DOI=10.1073/pnas.0831040100; PMCID=PMC153619
Jongeneel C.V., Iseli C., Stevenson B.J., Riggins G.J., Lal A., Mackay A., Harris R.A., O'Hare M.J., Neville A.M., Simpson A.J.G., Strausberg R.L.
Comprehensive sampling of gene expression in human cell lines with massively parallel signature sequencing.
Proc. Natl. Acad. Sci. U.S.A. 100:4702-4705(2003)
PubMed=12714694; DOI=10.1093/mutage/18.3.277
Yamada N.A., Castro A., Farber R.A.
Variation in the extent of microsatellite instability in human cell lines with defects in different mismatch repair genes.
Mutagenesis 18:277-282(2003)
PubMed=15748285; DOI=10.1186/1479-5876-3-11; PMCID=PMC555742
Adams S., Robbins F.-M., Chen D., Wagage D., Holbeck S.L., Morse H.C. 3rd, Stroncek D., Marincola F.M.
HLA class I and II genotype of the NCI-60 cell lines.
J. Transl. Med. 3:11.1-11.8(2005)
PubMed=15900046; DOI=10.1093/jnci/dji133
Mashima T., Oh-hara T., Sato S., Mochizuki M., Sugimoto Y., Yamazaki K., Hamada J.-i., Tada M., Moriuchi T., Ishikawa Y., Kato Y., Tomoda H., Yamori T., Tsuruo T.
p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target.
J. Natl. Cancer Inst. 97:765-777(2005)
PubMed=16418264; DOI=10.1073/pnas.0510146103; PMCID=PMC1327731
Liu Y., Bodmer W.F.
Analysis of p53 mutations and their expression in 56 colorectal cancer cell lines.
Proc. Natl. Acad. Sci. U.S.A. 103:976-981(2006)
PubMed=16854228; DOI=10.1186/1476-4598-5-29; PMCID=PMC1550420
Bandres Elizalde E.M., Cubedo E., Agirre X., Malumbres R., Zarate R., Ramirez N., Abajo A., Navarro A., Moreno I., Monzo M., Garcia-Foncillas J.
Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues.
Mol. Cancer 5:29.1-29.10(2006)
PubMed=17088437; DOI=10.1158/1535-7163.MCT-06-0433; PMCID=PMC2705832
Ikediobi O.N., Davies H.R., Bignell G.R., Edkins S., Stevens C., O'Meara S., Santarius T., Avis T., Barthorpe S., Brackenbury L., Buck G., Butler A.P., Clements J., Cole J., Dicks E., Forbes S., Gray K., Halliday K., Harrison R., Hills K., Hinton J., Hunter C., Jenkinson A., Jones D., Kosmidou V., Lugg R., Menzies A., Miroo T., Parker A., Perry J., Raine K.M., Richardson D., Shepherd R., Small A., Smith R., Solomon H., Stephens P.J., Teague J.W., Tofts C., Varian J., Webb T., West S., Widaa S., Yates A., Reinhold W.C., Weinstein J.N., Stratton M.R., Futreal P.A., Wooster R.
Mutation analysis of 24 known cancer genes in the NCI-60 cell line set.
Mol. Cancer Ther. 5:2606-2612(2006)
PubMed=17178751; DOI=10.1093/nar/gkl1030; PMCID=PMC1807964
Fiegler H., Geigl J.B., Langer S., Rigler D., Porter K., Unger K., Carter N.P., Speicher M.R.
High resolution array-CGH analysis of single cells.
Nucleic Acids Res. 35:e15.1-e15.10(2007)
PubMed=17363507; DOI=10.1158/1535-7163.MCT-06-0555
Wang J., Kuropatwinski K.K., Hauser J., Rossi M.R., Zhou Y.-F., Conway A., Kan J.L.C., Gibson N.W., Willson J.K.V., Cowell J.K., Brattain M.G.
Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis.
Mol. Cancer Ther. 6:1143-1150(2007)
PubMed=18258742; DOI=10.1073/pnas.0712176105; PMCID=PMC2268141
Emaduddin M., Bicknell D.C., Bodmer W.F., Feller S.M.
Cell growth, global phosphotyrosine elevation, and c-Met phosphorylation through Src family kinases in colorectal cancer cells.
Proc. Natl. Acad. Sci. U.S.A. 105:2358-2362(2008)
PubMed=18340113; DOI=10.4161/cbt.7.6.5838
Gongora C., Candeil L., Vezzio-Vie N., Copois V., Denis V., Bareil C., Molina F., Fraslon C., Conseiller E., Pau B., Martineau P., Del Rio M.
Altered expression of cell proliferation-related and interferon-stimulated genes in colon cancer cells resistant to SN38.
Cancer Biol. Ther. 7:822-832(2008)
PubMed=19372543; DOI=10.1158/1535-7163.MCT-08-0921; PMCID=PMC4020356
Lorenzi P.L., Reinhold W.C., Varma S., Hutchinson A.A., Pommier Y., Chanock S.J., Weinstein J.N.
DNA fingerprinting of the NCI-60 cell line panel.
Mol. Cancer Ther. 8:713-724(2009)
PubMed=19927377; DOI=10.1002/gcc.20730; PMCID=PMC2818350
Knutsen T., Padilla-Nash H.M., Wangsa D., Barenboim-Stapleton L., Camps J., McNeil N.E., Difilippantonio M.J., Ried T.
Definitive molecular cytogenetic characterization of 15 colorectal cancer cell lines.
Genes Chromosomes Cancer 49:204-223(2010)
PubMed=20164919; DOI=10.1038/nature08768; PMCID=PMC3145113
Bignell G.R., Greenman C.D., Davies H.R., Butler A.P., Edkins S., Andrews J.M., Buck G., Chen L., Beare D., Latimer C., Widaa S., Hinton J., Fahey C., Fu B.-Y., Swamy S., Dalgliesh G.L., Teh B.T., Deloukas P., Yang F.-T., Campbell P.J., Futreal P.A., Stratton M.R.
Signatures of mutation and selection in the cancer genome.
Nature 463:893-898(2010)
PubMed=20215515; DOI=10.1158/0008-5472.CAN-09-3458; PMCID=PMC2881662
Rothenberg S.M., Mohapatra G., Rivera M.N., Winokur D., Greninger P., Nitta M., Sadow P.M., Sooriyakumar G., Brannigan B.W., Ulman M.J., Perera R.M., Wang R., Tam A., Ma X.-J., Erlander M., Sgroi D.C., Rocco J.W., Lingen M.W., Cohen E.E.W., Louis D.N., Settleman J., Haber D.A.
A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers.
Cancer Res. 70:2158-2164(2010)
PubMed=20570890; DOI=10.1158/0008-5472.CAN-10-0192; PMCID=PMC2943514
Janakiraman M., Vakiani E., Zeng Z.-S., Pratilas C.A., Taylor B.S., Chitale D., Halilovic E., Wilson M., Huberman K., Ricarte Filho J.C.M., Persaud Y., Levine D.A., Fagin J.A., Jhanwar S.C., Mariadason J.M., Lash A., Ladanyi M., Saltz L.B., Heguy A., Paty P.B., Solit D.B.
Genomic and biological characterization of exon 4 KRAS mutations in human cancer.
Cancer Res. 70:5901-5911(2010)
PubMed=20606684; DOI=10.1038/sj.bjc.6605780; PMCID=PMC2920028
Bracht K., Nicholls A.M., Liu Y., Bodmer W.F.
5-fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency.
Br. J. Cancer 103:340-346(2010)
PubMed=22068913; DOI=10.1073/pnas.1111840108; PMCID=PMC3219108
Gillet J.-P., Calcagno A.M., Varma S., Marino M., Green L.J., Vora M.I., Patel C., Orina J.N., Eliseeva T.A., Singal V., Padmanabhan R., Davidson B., Ganapathi R., Sood A.K., Rueda B.R., Ambudkar S.V., Gottesman M.M.
Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance.
Proc. Natl. Acad. Sci. U.S.A. 108:18708-18713(2011)
PubMed=21912889; DOI=10.1007/s10637-011-9744-z
Sutherland H.S., Hwang I.Y., Marshall E.S., Lindsay B.S., Denny W.A., Gilchrist C., Joseph W.R., Greenhalgh D., Richardson E., Kestell P., Ding A., Baguley B.C.
Therapeutic reactivation of mutant p53 protein by quinazoline derivatives.
Invest. New Drugs 30:2035-2045(2012)
PubMed=22336246; DOI=10.1016/j.bmc.2012.01.017
Kong D.-X., Yamori T.
JFCR39, a panel of 39 human cancer cell lines, and its application in the discovery and development of anticancer drugs.
Bioorg. Med. Chem. 20:1947-1951(2012)
PubMed=22347499; DOI=10.1371/journal.pone.0031628; PMCID=PMC3276511
Ruan X.-Y., Kocher J.-P.A., Pommier Y., Liu H.-F., Reinhold W.C.
Mass homozygotes accumulation in the NCI-60 cancer cell lines as compared to HapMap trios, and relation to fragile site location.
PLoS ONE 7:E31628-E31628(2012)
PubMed=22384151; DOI=10.1371/journal.pone.0032096; PMCID=PMC3285665
Lee J.-S., Kim Y.K., Kim H.J., Hajar S., Tan Y.L., Kang N.-Y., Ng S.H., Yoon C.N., Chang Y.-T.
Identification of cancer cell-line origins using fluorescence image-based phenomic screening.
PLoS ONE 7:E32096-E32096(2012)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=22628656; DOI=10.1126/science.1218595; PMCID=PMC3526189
Jain M., Nilsson R., Sharma S., Madhusudhan N., Kitami T., Souza A.L., Kafri R., Kirschner M.W., Clish C.B., Mootha V.K.
Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation.
Science 336:1040-1044(2012)
PubMed=23272949; DOI=10.1186/1755-8794-5-66; PMCID=PMC3543849
Schlicker A., Beran G., Chresta C.M., McWalter G., Pritchard A., Weston S., Runswick S., Davenport S., Heathcote K., Castro D.A., Orphanides G., French T., Wessels L.F.A.
Subtypes of primary colorectal tumors correlate with response to targeted treatment in colorectal cell lines.
BMC Med. Genomics 5:66.1-66.15(2012)
PubMed=23546019; DOI=10.3892/ijo.2013.1868
Petitprez A., Poindessous V., Ouaret D., Regairaz M., Bastian G., Guerin E., Escargueil A.E., Larsen A.K.
Acquired irinotecan resistance is accompanied by stable modifications of cell cycle dynamics independent of MSI status.
Int. J. Oncol. 42:1644-1653(2013)
PubMed=23631600; DOI=10.1021/pr400260h
Loftus N.J., Lai L., Wilkinson R.W., Odedra R., Wilson I.D., Barnes A.J.
Global metabolite profiling of human colorectal cancer xenografts in mice using HPLC-MS/MS.
J. Proteome Res. 12:2980-2986(2013)
PubMed=23649806; DOI=10.1083/jcb.201210031; PMCID=PMC3653305
Kleiblova P., Shaltiel I.A., Benada J., Sevcik J., Pechackova S., Pohlreich P., Voest E.E., Dundr P., Bartek J., Kleibl Z., Medema R.H., Macurek L.
Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint.
J. Cell Biol. 201:511-521(2013)
PubMed=23671654; DOI=10.1371/journal.pone.0063056; PMCID=PMC3646030
Lu Y.-H., Soong T.D., Elemento O.
A novel approach for characterizing microsatellite instability in cancer cells.
PLoS ONE 8:E63056-E63056(2013)
PubMed=23856246; DOI=10.1158/0008-5472.CAN-12-3342; PMCID=PMC4893961
Abaan O.D., Polley E.C., Davis S.R., Zhu Y.-L.J., Bilke S., Walker R.L., Pineda M.A., Gindin Y., Jiang Y., Reinhold W.C., Holbeck S.L., Simon R.M., Doroshow J.H., Pommier Y., Meltzer P.S.
The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology.
Cancer Res. 73:4372-4382(2013)
PubMed=23933261; DOI=10.1016/j.celrep.2013.07.018
Moghaddas Gholami A., Hahne H., Wu Z.-X., Auer F.J., Meng C., Wilhelm M., Kuster B.
Global proteome analysis of the NCI-60 cell line panel.
Cell Rep. 4:609-620(2013)
PubMed=24042735; DOI=10.1038/oncsis.2013.35; PMCID=PMC3816225
Ahmed D., Eide P.W., Eilertsen I.A., Danielsen S.A., Eknaes M., Hektoen M., Lind G.E., Lothe R.A.
Epigenetic and genetic features of 24 colon cancer cell lines.
Oncogenesis 2:e71.1-e71.8(2013)
PubMed=24279929; DOI=10.1186/2049-3002-1-20; PMCID=PMC4178206
Dolfi S.C., Chan L.L.-Y., Qiu J., Tedeschi P.M., Bertino J.R., Hirshfield K.M., Oltvai Z.N., Vazquez A.
The metabolic demands of cancer cells are coupled to their size and protein synthesis rates.
Cancer Metab. 1:20.1-20.13(2013)
PubMed=24670534; DOI=10.1371/journal.pone.0092047; PMCID=PMC3966786
Varma S., Pommier Y., Sunshine M., Weinstein J.N., Reinhold W.C.
High resolution copy number variation data in the NCI-60 cancer cell lines from whole genome microarrays accessible through CellMiner.
PLoS ONE 9:E92047-E92047(2014)
PubMed=24755471; DOI=10.1158/0008-5472.CAN-14-0013
Mouradov D., Sloggett C., Jorissen R.N., Love C.G., Li S., Burgess A.W., Arango D., Strausberg R.L., Buchanan D., Wormald S., O'Connor L., Wilding J.L., Bicknell D.C., Tomlinson I.P.M., Bodmer W.F., Mariadason J.M., Sieber O.M.
Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer.
Cancer Res. 74:3238-3247(2014)
PubMed=25960936; DOI=10.4161/21624011.2014.954893; PMCID=PMC4355981
Boegel S., Lower M., Bukur T., Sahin U., Castle J.C.
A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines.
OncoImmunology 3:e954893.1-e954893.12(2014)
PubMed=25984343; DOI=10.1038/sdata.2014.35; PMCID=PMC4432652
Cowley G.S., Weir B.A., Vazquez F., Tamayo P., Scott J.A., Rusin S., East-Seletsky A., Ali L.D., Gerath W.F.J., Pantel S.E., Lizotte P.H., Jiang G.-Z., Hsiao J., Tsherniak A., Dwinell E., Aoyama S., Okamoto M., Harrington W., Gelfand E.T., Green T.M., Tomko M.J., Gopal S., Wong T.C., Li H.-B., Howell S., Stransky N., Liefeld T., Jang D., Bistline J., Meyers B.H., Armstrong S.A., Anderson K.C., Stegmaier K., Reich M., Pellman D., Boehm J.S., Mesirov J.P., Golub T.R., Root D.E., Hahn W.C.
Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies.
Sci. Data 1:140035-140035(2014)
PubMed=25485619; DOI=10.1038/nbt.3080
Klijn C., Durinck S., Stawiski E.W., Haverty P.M., Jiang Z.-S., Liu H.-B., Degenhardt J., Mayba O., Gnad F., Liu J.-F., Pau G., Reeder J., Cao Y., Mukhyala K., Selvaraj S.K., Yu M.-M., Zynda G.J., Brauer M.J., Wu T.D., Gentleman R.C., Manning G., Yauch R.L., Bourgon R., Stokoe D., Modrusan Z., Neve R.M., de Sauvage F.J., Settleman J., Seshagiri S., Zhang Z.-M.
A comprehensive transcriptional portrait of human cancer cell lines.
Nat. Biotechnol. 33:306-312(2015)
PubMed=25576301; DOI=10.1074/mcp.M114.042812; PMCID=PMC4349985
Bassani-Sternberg M., Pletscher-Frankild S., Jensen L.J., Mann M.
Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation.
Mol. Cell. Proteomics 14:658-673(2015)
PubMed=25841592; DOI=10.1016/j.jprot.2015.03.019
Piersma S.R., Knol J.C., de Reus I., Labots M., Sampadi B.K., Pham T.V., Ishihama Y., Verheul H.M.W., Jimenez C.R.
Feasibility of label-free phosphoproteomics and application to base-line signaling of colorectal cancer cell lines.
J. Proteomics 127:247-258(2015)
PubMed=25877200; DOI=10.1038/nature14397
Yu M., Selvaraj S.K., Liang-Chu M.M.Y., Aghajani S., Busse M., Yuan J., Lee G., Peale F.V., Klijn C., Bourgon R., Kaminker J.S., Neve R.M.
A resource for cell line authentication, annotation and quality control.
Nature 520:307-311(2015)
PubMed=25926053; DOI=10.1038/ncomms8002
Medico E., Russo M., Picco G., Cancelliere C., Valtorta E., Corti G., Buscarino M., Isella C., Lamba S., Martinoglio B., Veronese S., Siena S., Sartore-Bianchi A., Beccuti M., Mottolese M., Linnebacher M., Cordero F., Di Nicolantonio F., Bardelli A.
The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets.
Nat. Commun. 6:7002.1-7002.10(2015)
PubMed=25944804; DOI=10.1158/1078-0432.CCR-14-2457
Bazzocco S., Dopeso H., Carton-Garcia F., Macaya I., Andretta E., Chionh F., Rodrigues P., Garrido M., Alazzouzi H., Nieto R., Sanchez A., Schwartz S. Jr., Bilic J., Mariadason J.M., Arango D.
Highly expressed genes in rapidly proliferating tumor cells as new targets for colorectal cancer treatment.
Clin. Cancer Res. 21:3695-3704(2015)
PubMed=26169745; DOI=10.1186/s12967-015-0576-z; PMCID=PMC4499939
Halama A., Guerrouahen B.S., Pasquier J., Diboun I., Karoly E.D., Suhre K., Rafii A.
Metabolic signatures differentiate ovarian from colon cancer cell lines.
J. Transl. Med. 13:223.1-223.12(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5; PMCID=PMC4653878
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26719794; DOI=10.1186/s13742-015-0106-1; PMCID=PMC4696294
Teo A.S.M., Verzotto D., Yao F., Nagarajan N., Hillmer A.M.
Single-molecule optical genome mapping of a human HapMap and a colorectal cancer cell line.
GigaScience 4:65.1-65.6(2015)
PubMed=26537799; DOI=10.1074/mcp.M115.051235; PMCID=PMC4762531
Holst S., Deuss A.J.M., van Pelt G.W., van Vliet S.J., Garcia-Vallejo J.J., Koeleman C.A.M., Deelder A.M., Mesker W.E., Tollenaar R.A.E.M., Rombouts Y., Wuhrer M.
N-glycosylation profiling of colorectal cancer cell lines reveals association of fucosylation with differentiation and caudal type homebox 1 (CDX1)/villin mRNA expression.
Mol. Cell. Proteomics 15:124-140(2016)
PubMed=27377824; DOI=10.1038/sdata.2016.52; PMCID=PMC4932877
Mestdagh P., Lefever S., Volders P.-J., Derveaux S., Hellemans J., Vandesompele J.
Long non-coding RNA expression profiling in the NCI60 cancer cell line panel using high-throughput RT-qPCR.
Sci. Data 3:160052-160052(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27807467; DOI=10.1186/s13100-016-0078-4; PMCID=PMC5087121
Zampella J.G., Rodic N., Yang W.R., Huang C.R.L., Welch J., Gnanakkan V.P., Cornish T.C., Boeke J.D., Burns K.H.
A map of mobile DNA insertions in the NCI-60 human cancer cell panel.
Mob. DNA 7:20.1-20.11(2016)
PubMed=28179481; DOI=10.1158/1535-7163.MCT-16-0578
Tanaka N., Mashima T., Mizutani A., Sato A., Aoyama A., Gong B., Yoshida H., Muramatsu Y., Nakata K., Matsuura M., Katayama R., Nagayama S., Fujita N., Sugimoto Y., Seimiya H.
APC mutations as a potential biomarker for sensitivity to tankyrase inhibitors in colorectal cancer.
Mol. Cancer Ther. 16:752-762(2017)
PubMed=28192450; DOI=10.1371/journal.pone.0171435; PMCID=PMC5305277
Fasterius E., Raso C., Kennedy S.A., Rauch N., Lundin P., Kolch W., Uhlen M., Al-Khalili Szigyarto C.
A novel RNA sequencing data analysis method for cell line authentication.
PLoS ONE 12:E0171435-E0171435(2017)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=28601559; DOI=10.1016/j.cels.2017.05.009; PMCID=PMC5493283
Bekker-Jensen D.B., Kelstrup C.D., Batth T.S., Larsen S.C., Haldrup C., Bramsen J.B., Sorensen K.D., Hoyer S., Orntoft T.F., Lindbjerg Andersen C., Nielsen M.L., Olsen J.V.
An optimized shotgun strategy for the rapid generation of comprehensive human proteomes.
Cell Syst. 4:587-599.e4(2017)
PubMed=28683746; DOI=10.1186/s12943-017-0691-y; PMCID=PMC5498998
Berg K.C.G., Eide P.W., Eilertsen I.A., Johannessen B., Bruun J., Danielsen S.A., Bjornslett M., Meza-Zepeda L.A., Eknaes M., Lind G.E., Myklebost O., Skotheim R.I., Sveen A., Lothe R.A.
Multi-omics of 34 colorectal cancer cell lines -- a resource for biomedical studies.
Mol. Cancer 16:116.1-116.16(2017)
PubMed=28854368; DOI=10.1016/j.celrep.2017.08.010; PMCID=PMC5583477
Roumeliotis T.I., Williams S.P., Goncalves E., Alsinet C., Del Castillo Velasco-Herrera M., Aben N., Ghavidel F.Z., Michaut M., Schubert M., Price S., Wright J.C., Yu L., Yang M., Dienstmann R., Guinney J.H., Beltrao P., Brazma A., Pardo M., Stegle O., Adams D.J., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Choudhary J.S.
Genomic determinants of protein abundance variation in colorectal cancer cells.
Cell Rep. 20:2201-2214(2017)
PubMed=29101300; DOI=10.15252/msb.20177701; PMCID=PMC5731344
Frejno M., Zenezini Chiozzi R., Wilhelm M., Koch H., Zheng R.-S., Klaeger S., Ruprecht B., Meng C., Kramer K., Jarzab A., Heinzlmeir S., Johnstone E., Domingo E., Kerr D.J., Jesinghaus M., Slotta-Huspenina J., Weichert W., Knapp S., Feller S.M., Kuster B.
Pharmacoproteomic characterisation of human colon and rectal cancer.
Mol. Syst. Biol. 13:951-951(2017)
PubMed=29207035; DOI=10.3892/ijo.2017.4206
Olejniczak A., Szarynska M., Kmiec Z.
In vitro characterization of spheres derived from colorectal cancer cell lines.
Int. J. Oncol. 52:599-612(2018)
PubMed=29444439; DOI=10.1016/j.celrep.2018.01.051; PMCID=PMC6343826
Yuan T.L., Amzallag A., Bagni R., Yi M., Afghani S., Burgan W., Fer N., Strathern L.A., Powell K., Smith B., Waters A.M., Drubin D.A., Thomson T., Liao R., Greninger P., Stein G.T., Murchie E., Cortez E., Egan R.K., Procter L., Bess M., Cheng K.T., Lee C.-S., Lee L.C., Fellmann C., Stephens R., Luo J., Lowe S.W., Benes C.H., McCormick F.
Differential effector engagement by oncogenic KRAS.
Cell Rep. 22:1889-1902(2018)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)
PubMed=30971826; DOI=10.1038/s41586-019-1103-9
Behan F.M., Iorio F., Picco G., Goncalves E., Beaver C.M., Migliardi G., Santos R., Rao Y., Sassi F., Pinnelli M., Ansari R., Harper S., Jackson D.A., McRae R., Pooley R., Wilkinson P., van der Meer D.J., Dow D., Buser-Doepner C.A., Bertotti A., Trusolino L., Stronach E.A., Saez-Rodriguez J., Yusa K., Garnett M.J.
Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens.
Nature 568:511-516(2019)
PubMed=31068700; DOI=10.1038/s41586-019-1186-3; PMCID=PMC6697103
Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R. 3rd, Barretina J.G., Gelfand E.T., Bielski C.M., Li H.-X., Hu K., Andreev-Drakhlin A.Y., Kim J., Hess J.M., Haas B.J., Aguet F., Weir B.A., Rothberg M.V., Paolella B.R., Lawrence M.S., Akbani R., Lu Y.-L., Tiv H.L., Gokhale P.C., de Weck A., Mansour A.A., Oh C., Shih J., Hadi K., Rosen Y., Bistline J., Venkatesan K., Reddy A., Sonkin D., Liu M., Lehar J., Korn J.M., Porter D.A., Jones M.D., Golji J., Caponigro G., Taylor J.E., Dunning C.M., Creech A.L., Warren A.C., McFarland J.M., Zamanighomi M., Kauffmann A., Stransky N., Imielinski M., Maruvka Y.E., Cherniack A.D., Tsherniak A., Vazquez F., Jaffe J.D., Lane A.A., Weinstock D.M., Johannessen C.M., Morrissey M.P., Stegmeier F., Schlegel R., Hahn W.C., Getz G., Mills G.B., Boehm J.S., Golub T.R., Garraway L.A., Sellers W.R.
Next-generation characterization of the Cancer Cell Line Encyclopedia.
Nature 569:503-508(2019)
PubMed=31978347; DOI=10.1016/j.cell.2019.12.023; PMCID=PMC7339254
Nusinow D.P., Szpyt J., Ghandi M., Rose C.M., McDonald E.R. 3rd, Kalocsay M., Jane-Valbuena J., Gelfand E.T., Schweppe D.K., Jedrychowski M.P., Golji J., Porter D.A., Rejtar T., Wang Y.K., Kryukov G.V., Stegmeier F., Erickson B.K., Garraway L.A., Sellers W.R., Gygi S.P.
Quantitative proteomics of the Cancer Cell Line Encyclopedia.
Cell 180:387-402.e16(2020)
PubMed=32172478; DOI=10.1007/s12253-020-00805-3
Xu Y.-T., Zhang L., Wang Q.-L., Zheng M.-J.
Comparison of different colorectal cancer with liver metastases models using six colorectal cancer cell lines.
Pathol. Oncol. Res. 26:2177-2183(2020)
PubMed=32927768; DOI=10.3390/cancers12092582; PMCID=PMC7564713
Schulte am Esch J., Windmoller B.A., Hanewinkel J., Storm J., Forster C., Wilkens L., Kruger M., Kaltschmidt B., Kaltschmidt C.
Isolation and characterization of two novel colorectal cancer cell lines, containing a subpopulation with potential stem-like properties: treatment options by MYC/NMYC inhibition.
Cancers (Basel) 12:2582.1-2582.34(2020)"