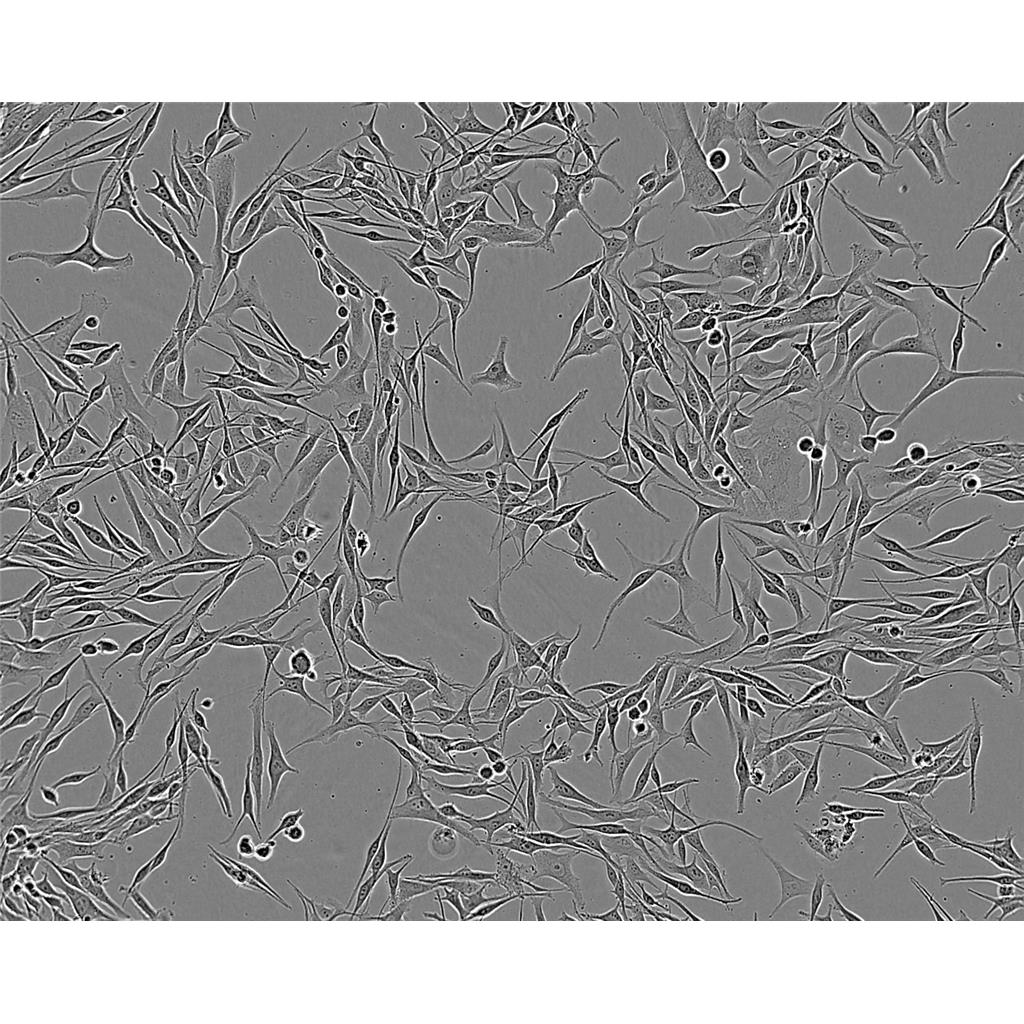
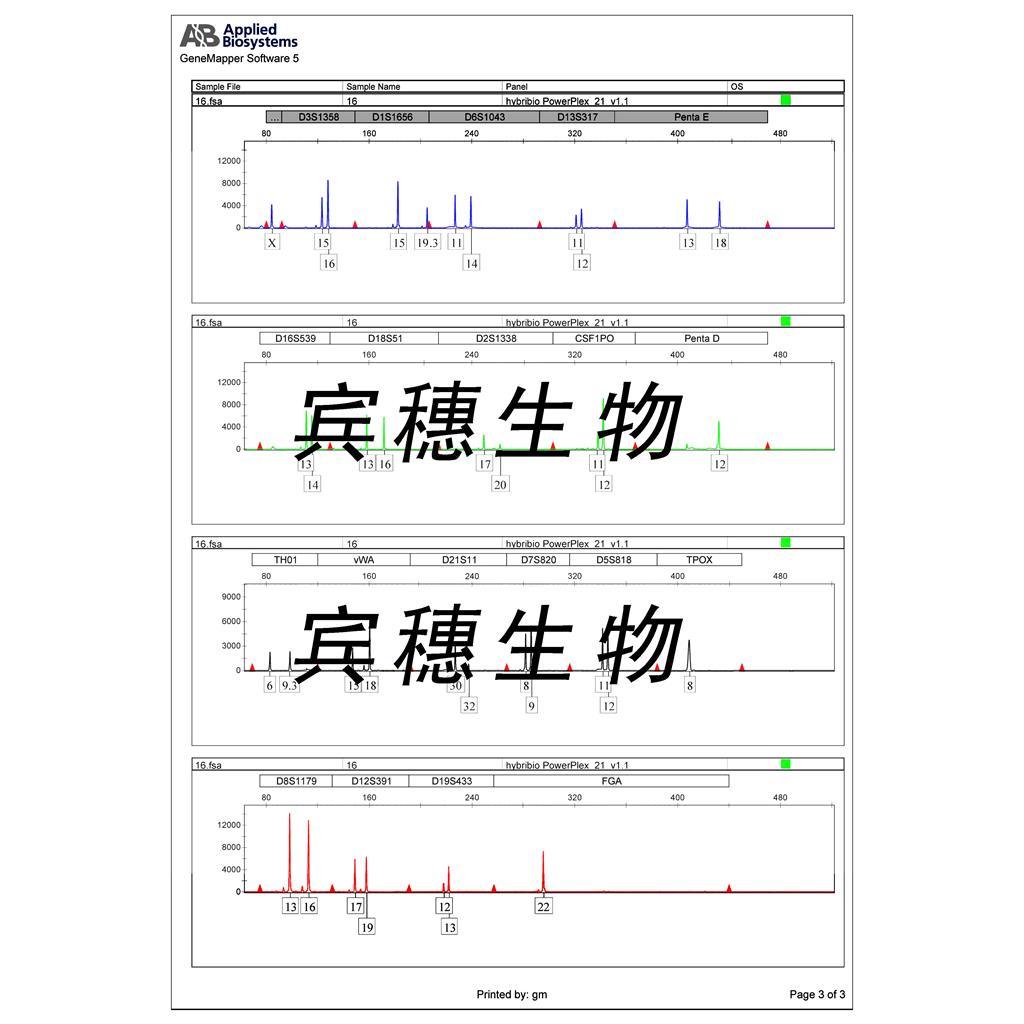
"Raji人Burkitt's淋巴瘤细胞代次低|培养基|送STR图谱
传代比例:1:2-1:4(首次传代建议1:2)
生长特性:悬浮生长
细胞系的应用:1)免疫组化研究2)RNA干扰研究3)药物作用研究4)慢病毒转染研究等其它应用。细胞系通常用于实验研究,如增殖、迁移、侵袭等。细胞系在多个领域的研究中被广泛应用,包括基础医学、临床试验、药物筛选和分子生物学研究。这些研究不仅在中国,也在日本、美国和欧洲等多个国家和地区进行。
换液周期:每周2-3次
MC 3T3-E1 Cells;背景说明:该细胞有多个亚克隆,可以作为体外研究成骨细胞分化的良好模型,尤其是ECM信号通路的作用。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:成纤维细胞样;相关产品有:SUNE1细胞、WBF344细胞、OE19细胞
CNE2Z Cells;背景说明:鼻咽癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:LOU-NH-91细胞、COS-1细胞、CNE细胞
Glioma 261 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:上皮细胞样;相关产品有:A431细胞、KYSE450细胞、WM 115细胞
Raji人Burkitt's淋巴瘤细胞代次低|培养基|送STR图谱
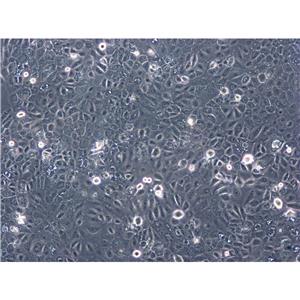
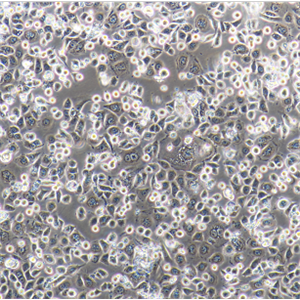
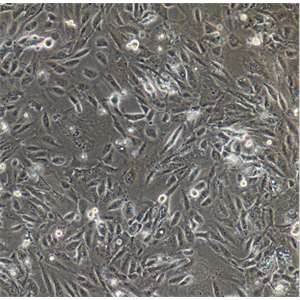
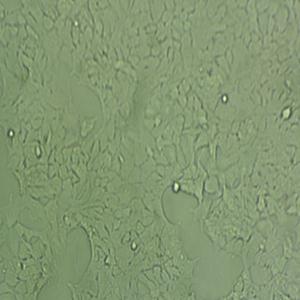
背景信息:Raji细胞由PulvertaftRJV于1963年从一位11岁黑人男孩的左上颌骨的Burkitt淋巴瘤中分离建立的,是第一个人类造血系统的连续传代细胞,为B细胞起源。该细胞中含有EBV,需要在二级生物安全柜中操作;可作转染宿主。
┈订┈购(技术服务)┈热┈线:1┈3┈6┈4┈1┈9┈3┈0┈7┈9┈1【微信同号】┈Q┈Q:3┈1┈8┈0┈8┈0┈7┈3┈2┈4;
ATCC细胞库(American Type Culture Colection),该中心一直致力于细胞分类、鉴定和保藏工作。ATCC是全球最大的生物资源保藏中心,ATCC通过行业标准产品、服务和创新解决方案支持全球学术、政府、生物技术、制药、食品、农业和工业领域的科学进步。ATCC提供的服务和定制解决方案包括细胞和微生物培养、鉴定、生物衍生物的开发和生产、性能测试和生物资源保藏服务。美国国家标准协会(ANSI)认可了ATCC标准开发组织,并制定了标准协议,以确保生物材料的可靠性和可重复性。ATCC的使命是为了获取、鉴定、保存、开发、标准化和分发生物资源和生物信息,以提高和应用生物科学知识。
产品包装:复苏发货:T25培养瓶(一瓶)或冻存发货:1ml冻存管(两支)
来源说明:细胞主要来源ATCC、ECACC、DSMZ、RIKEN等细胞库
Raji人Burkitt's淋巴瘤细胞代次低|培养基|送STR图谱
物种来源:人源、鼠源等其它物种来源
LC-1/sq Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:Panc 10.05细胞、Scott细胞、EC-GI细胞
DoHH-2 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MSCs(mUCMSCs)细胞、Lung cancer-1/squamous细胞、RAW264.7细胞
IOSE-29 Cells;背景说明:卵巢;上皮细胞;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:LTEP-a-2细胞、SNU-C2B细胞、MAntle cell VERona-1细胞
NCI-H1436 Cells;背景说明:详见相关文献介绍;传代方法:随细胞的密度而增加;生长特性:悬浮生长;形态特性:详见产品说明书;相关产品有:LNCaP FGC细胞、V79细胞、H2087细胞
┈订┈购(技术服务)┈热┈线:1┈3┈6┈4┈1┈9┈3┈0┈7┈9┈1【微信同号】┈Q┈Q:3┈1┈8┈0┈8┈0┈7┈3┈2┈4;
形态特性:淋巴母细胞样
贴壁消化难题:1,先用PBS 把细胞洗两遍,使瓶内没有血清了,减少对胰酶的中和,然后用新配的0.25%的胰酶加入3ml左右,放在37度,然后可以在细胞有些消化下来时,拿着瓶口,运用手腕的力量轻轻震荡瓶内体,这样细胞很快就下来了,还不需要吹打,分散也均匀;2,成团、絮状:消化里加入eda可以减少细胞成团的现象,血清可以终止胰酶的作用,如果是进口血清的话也能终止eda的作用。用胰酶消化后胰酶可以倒掉,也可以不倒,直接加血清终止,如果消化中加入了eda的话,就要将消化倒干净,如果细胞贴壁要求不是很严格的话,一般不需要进行离心。鼻咽癌细胞的贴壁能力很强,用0.5%胰酶(含0.1%EDA)一般要消化12~15min。用PBS洗涤时要洗净残余的培养基,加入胰酶后在培养箱中消化(避免细胞室温下受损以及在此温度时胰酶活性Zui强)至细胞收缩变圆(可显微镜下观察)且有少许细胞脱落(有流下来的趋势),随后立即弃去胰酶(如果脱落的细胞很多且需要大量细胞实验,则不能弃去胰酶),加入培养基仔细吹打(不能用无血清培养基或者PBS替代,否则细胞聚集成团块或絮状)。一般我都离心一次弃去上清(去除残留的胰酶及漂浮的死细胞或细胞碎片);消化过度:马上用培养基中和,用吸管吹打细胞,收集全部的细胞到以无菌的离心管中800RPM 3分钟。弃上清,用全培重悬,换新的培养瓶继续培养,状态不HAO的细胞在培养的过程中会死亡脱落,在换的时候可以清除掉!
LA-N-6 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:NCIH1781细胞、HLEC-SRA 01/04细胞、KLN 205细胞
SVGp12 Cells;背景说明:星形胶质细胞;SV40转化;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:H-2066细胞、SW-1573细胞、TE354T细胞
RA-FLSs Cells;背景说明:关节;成纤维 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:HCC0044细胞、ZR75-30细胞、L6细胞
OVSAHO Cells;背景说明:卵巢癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:WM-266-mel细胞、Hs683T细胞、UWB1.289+BRCA1细胞
PaTu8988s Cells;背景说明:胰腺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Hs863T细胞、HCEC-B4G12细胞、HPBALL细胞
NCI-H1688 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MOLT3细胞、P 3 HR 1细胞、CCD 1112SK细胞
H920 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:HG2855细胞、SPCA-1细胞、MA-c细胞
MV4:11 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:P30OHK细胞、K1细胞、BXPC3细胞
CCRFCEM Cells;背景说明:G.E. Foley 等人建立了类淋巴母细胞细胞株CCRF-CEM。 细胞是1964年11月从一位四岁白人女性急性淋巴细胞白血病患者的外周血白血球衣中得到。此细胞系从香港收集而来。;传代方法:1:2传代。3天内可长满。;生长特性:悬浮生长;形态特性:淋巴母细胞样;相关产品有:MM-1S细胞、SR786细胞、HTori:3细胞
HPBALL Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:HBL100细胞、FDCP1细胞、3T3-F442A细胞
DV-90 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:Eca-109细胞、Wayne State University-Head and Neck 13细胞、Duck embryo细胞
DC2.4 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明书;相关产品有:Psi-2-DAP细胞、PK136细胞、HNSC细胞
NS-1 Cells;背景说明:这是P3X63Ag8(ATCCTIB-9)的一个不分泌克隆。Kappa链合成了但不分泌。能抗0.1mM8-氮杂鸟嘌呤但不能在HAT培养基中生长。据报道它是由于缺失了3-酮类固醇还原酶活性的胆固醇营养缺陷型。检测表明肢骨发育畸形病毒(鼠痘)阴性。;传代方法:1:2传代,3天内可长满。;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:MADB 106细胞、MLOY4细胞、KYSE-140细胞
WRL68 Cells;背景说明:胚胎;肝 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:D-283细胞、H-2107细胞、LC-2/ad细胞
PTK 2 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:COLO 699细胞、NS1-Ag4/1细胞、hCMEC/D3细胞
KYSE 270 Cells;背景说明:详见相关文献介绍;传代方法:1:5传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:HOP92细胞、Hs739T细胞、G-361细胞
Det. 562 Cells;背景说明:器官:咽头 疾病:癌 取材转移灶:胸水;传代方法:1:2-1:4传代,2-3天换液1次。;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:H19-7细胞、SNU620细胞、MES 13细胞
40L Cells(提供STR鉴定图谱)
Abcam Jurkat NDUFAF2 KO Cells(提供STR鉴定图谱)
ALZ.14 Cells(提供STR鉴定图谱)
BayGenomics ES cell line RRK057 Cells(提供STR鉴定图谱)
BayGenomics ES cell line XL034 Cells(提供STR鉴定图谱)
CADO-LC10 Cells(提供STR鉴定图谱)
DA00476 Cells(提供STR鉴定图谱)
DA04819 Cells(提供STR鉴定图谱)
FT-1 Cells(提供STR鉴定图谱)
MOLP-2 Cells;背景说明:骨髓瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:NCI-H157细胞、H-510A细胞、HCC-2935细胞
Raji人Burkitt's淋巴瘤细胞代次低|培养基|送STR图谱
HCC366 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:L-363细胞、KYSE70细胞、GM00637H细胞
CORL105 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:HT-29细胞、Ontario Cancer Institute-Acute Myeloid Leukemia-3细胞、MDA-MB 361细胞
GM07404 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明书;相关产品有:639-V细胞、Pa017C细胞、OCI/AML-5细胞
Human Kidney-2 Cells;背景说明:该细胞属源于正常肾的近曲小管细胞,通过导入HPV-16 E6/E7基因而获得永生化。将含有HPV-16 E6/E7基因的重组的逆转录病毒载体pLXSN 16 E6/E7转染外生包装细胞Psi-2,Psi-2细胞产生的病毒再去感染兼嗜性包装细胞系PA317,最后将PA317产生的病毒颗粒导入正常的肾皮质近曲小管细胞。尽管pLXSN 16 E6/E7中含有新霉素抗性,但未用G418筛选转导克隆。Southern和FISH分析显示HK-2细胞来源于单克隆。PCR检测证实HK-2细胞基因组中含有E6/E7基因。;传代方法:1:4传代;2-3天换液1次;生长特性:贴壁生长;形态特性:上皮样;相关产品有:NCI-H1975细胞、B16-F10细胞、H-1876细胞
NFS 60 Cells;背景说明:详见相关文献介绍;传代方法:1:3传代;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:CHO细胞、NCI-SNU-761细胞、Malme3M细胞
123-10 Cells(提供STR鉴定图谱)
S16 Cells;背景说明:Schwann细胞;自发永生;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:MS751细胞、Hep 3B2细胞、BEL-7404细胞
MNNG/HOS Cells;背景说明:骨肉瘤;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:H2195细胞、Hs-852-T细胞、OCI-LY-3细胞
SNU-251 Cells;背景说明:卵巢内膜癌;腹水转移;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:NSC-34细胞、751-NA-15细胞、CPA 47细胞
BEL-7404 Cells;背景说明:用Northernblot方法,未能检测到细胞中1.3kbLFIRE-1/HFREP-1mRNA的表达。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:CO115细胞、RPMI.8226细胞、High5细胞
HOSEpiC Cells;背景说明:卵巢;上皮 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Toledo细胞、Hs-27细胞、P-36细胞
RAW2647 Cells;背景说明:单核巨噬细胞白血病;雄性;BALB/c;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:P30/OHK细胞、0V-1063细胞、SNU520细胞
NU-GC-4 Cells;背景说明:胃癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:半贴壁;形态特性:详见产品说明书;相关产品有:TK-1细胞、Ramos G6.C10细胞、BERH-2细胞
B-3 Cells;背景说明:晶状体;Ad12-SV40转化;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:COLO-738细胞、Hs578Bst细胞、H-2452细胞
GM21070 Cells(提供STR鉴定图谱)
HAP1 LIMCH1 (-) 2 Cells(提供STR鉴定图谱)
HEL92.1.7 Cells;背景说明:详见相关文献介绍;传代方法:每周2-3次。;生长特性:悬浮生长;形态特性:成淋巴细胞;相关产品有:MLMEC细胞、MDA157细胞、GM05372细胞
H-1876 Cells;背景说明:详见相关文献介绍;传代方法:每周换液2次。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:DHL-6细胞、SNU-182细胞、AML-193细胞
NCIH1417 Cells;背景说明:小细胞肺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:SUP-T1细胞、E.L.4细胞、Panc 2.03细胞
Tca8113 Cells;背景说明:舌鳞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Hs 695T细胞、SUDHL-5细胞、Tu-212细胞
SNB-19 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明书;相关产品有:LP1细胞、CCD18细胞、SKOV3细胞
LC-1/sq Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:Panc 10.05细胞、Scott细胞、EC-GI细胞
SKG IIIa Cells;背景说明:详见相关文献介绍;传代方法:2x10^4 cells/ml;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:HS578T细胞、NIH:OVCAR-3细胞、WEHI 3细胞
MCF/Adr Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:BE(2)M-17细胞、BEL-7404细胞、CCC-ESF-1细胞
HPSI0216i-boho_3 Cells(提供STR鉴定图谱)
K562 eGFP-KLF13 Cells(提供STR鉴定图谱)
mEC25 Cells(提供STR鉴定图谱)
NH50161 Cells(提供STR鉴定图谱)
QG-90 Cells(提供STR鉴定图谱)
TRL 4 Cells(提供STR鉴定图谱)
UKF-NB-3rMEL2000 Cells(提供STR鉴定图谱)
HAP1 USP11 (-) 3 Cells(提供STR鉴定图谱)
NCIH146 Cells;背景说明:详见相关文献介绍;传代方法:1:2—1:6传代,每周换液2-3次;生长特性:悬浮生长;形态特性:上皮细胞;相关产品有:NCI-H64细胞、A375SM细胞、293-F细胞
CEM-T4 Cells;背景说明:急性T淋巴细胞白血病;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:MDA-MB 468细胞、OCI-AML-2细胞、MDCK (NBL-2)细胞
FT-293 Cells;背景说明:该细胞稳定表达SV40大T抗原,并且促进最适病毒产物的产生。;传代方法:1:2传代;生长特性:悬浮生长;形态特性:圆形;相关产品有:B104 [Rat neuroblastoma]细胞、ARH-77细胞、NPA87-1细胞
TGBC-11-TKB Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:RPMI7951细胞、J82 COT细胞、LC-1 sq细胞
P31-FUJ Cells;背景说明:详见相关文献介绍;传代方法:1:5传代;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:801-D细胞、HCT 15细胞、SL-29细胞
P31-FUJ Cells;背景说明:详见相关文献介绍;传代方法:1:5传代;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:801-D细胞、HCT 15细胞、SL-29细胞
B 95.8 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明书;相关产品有:NS20Y细胞、KBM5细胞、HFE-145细胞
SK-RC-20 Cells;背景说明:肾癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:CT 26细胞、L 1210细胞、TE 32.T细胞
L-6 myoblast Cells;背景说明:该细胞是Yaffe在甲基胆蒽存在的情况下从大鼠大腿肌原代培养的最初两代细胞中分离得到的;在培养基中融合形成多核的肌管和横纹肌纤维,细胞融合的程度随着代数的增加而下降,因此细胞应低代次冷冻并周期性地重新克隆以选择融合能力强的细胞。鼠痘病毒阴性。该细胞应在达汇合状态前传代,以延缓细胞分化能力的丧失。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:成肌细胞样;相关产品有:P30-OHKUBO细胞、SKCO-1细胞、TALL-104细胞
GM03190 Cells;背景说明:1967年,该细胞系KleinE和KleinG建系,源于一名16岁患有Burkitt's淋巴瘤的黑人男性,beta-2-微球蛋白阴性,表达EBNA,VCA,sIg。该细胞携带EB病毒,是一个典型的B淋巴母细胞系,可用于白血病发病机制的研究。;传代方法:1:2传代;生长特性:悬浮生长;形态特性:淋巴母细胞样;相关产品有:H-1092细胞、H69细胞、WiDr/S细胞
P3HR1-BL Cells;背景说明:详见相关文献介绍;传代方法:每2-3天换液;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:Pan02细胞、MCA-205细胞、SK-ES-1细胞
P3J-HR-1 Cells;背景说明:详见相关文献介绍;传代方法:每2-3天换液;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:HFF1细胞、HIEC6细胞、MCF7-GFP细胞
KNS42 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:多边形;相关产品有:MV4II细胞、HCC0095细胞、NCI-H526细胞
H4-II-EC3 Cells;背景说明:在糖皮质激素、胰岛素或cAMP衍生物的诱导下可以产生酪酸基转移酶;可被逆转录病毒感染;可产生白蛋白、转铁蛋白、凝血酶原;在AxC大鼠中可以成瘤。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:SUM-190细胞、SKRC-20细胞、T241细胞
UPCI:SCC90 Cells;背景说明:舌鳞癌细胞;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:Jurkat (clone E6-1)细胞、NCIH1155细胞、GM00637B细胞
SPH-7(T) Cells(提供STR鉴定图谱)
H-1238 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:ARH77细胞、Anip[973]细胞、THLE-3细胞
T84 Cells;背景说明:T84细胞株是从一位72岁男性结肠癌患者的肺转移灶建立的可移植人类癌细胞株。 肿瘤组织皮下接种于BALB/c裸鼠,并连续进行移植。 [26072] 在裸鼠身上的移植过程中,细胞株始终保持结肠癌的原始组织性状。 [26072] 在无胸腺小鼠中传代23代后建立了T84细胞株。 这些细胞单层生长到饱和并在接触细胞间展现出紧密连接和桥粒。 [1155] 有很多关于多肽类激素和神经递质并维持定向电解质传输的受体。 [1155] 这株细胞展现了接触细胞中的紧密连接和桥粒。 [1155] 角蛋白免疫过氧化物酶染色阳性。;传代方法:1:2-1:4传代;每周2次。;生长特性:贴壁生长;形态特性:上皮细胞,多角;相关产品有:A-2780细胞、KU 812细胞、GTL16细胞
SuDHL 1 Cells;背景说明:间变性大细胞淋巴瘤;胸腔积液转移;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:HSKMC细胞、BE2M17细胞、NCIH3255细胞
EA. hy 926 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:SUM190PT细胞、THLE-2细胞、AU 565细胞
U 138 MG Cells;背景说明:星形细胞瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:A375-MEL细胞、R-1059-D细胞、32D-Cl3细胞
TALL1 Cells;背景说明:该细胞源于一名复发T-ALL(急性T淋巴细胞性白血病)的儿童的外周血;具有很强的细胞毒性,体内体外实验中都能破坏肿瘤细胞;IL-2可使细胞更好地生长;α/β TCR阳性,γ/δ TCR阴性;可产生IFNγ、TNF-α和GM-CSF。;传代方法:维持细胞密度在4×105-1×106 cells/ml之间,2-3天换液1次 ;生长特性:悬浮生长;形态特性:淋巴母细胞;相关产品有:VERO76细胞、293-H细胞、SHZ-88细胞
Raji人Burkitt's淋巴瘤细胞代次低|培养基|送STR图谱
NCIH1048 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:8传代;;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:SW-1573细胞、OCIAML5细胞、MDCC-MSB1细胞
Hs-578T Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:MV411细胞、SGC-996细胞、H-2126细胞
FRO 81-2 Cells;背景说明:未分化甲状腺癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:hTERT-RPE1细胞、Ect1/E6E7细胞、IMCD3细胞
MuM-2B Cells;背景说明:脉络膜黑色素瘤;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明书;相关产品有:MNNG细胞、CT26-clone 25细胞、LTEP-sm细胞
SU-DHL-10 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:淋巴母细胞;相关产品有:BC-020细胞、NE-1细胞、IOSE 29细胞
NCIH196 Cells;背景说明:详见相关文献介绍;传代方法:1:4-1:6传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:详见产品说明书;相关产品有:YES 2细胞、C4I细胞、BJ [Human fibroblast]细胞
HD-LM-2 Cells;背景说明:霍奇金淋巴瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明书;相关产品有:MIO-M1细胞、BRL3A细胞、CT26.WT细胞
RBMVEC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明书部分;形态特性:详见产品说明书;相关产品有:NCI-H1882细胞、SK-N-BE(2C)细胞、PTK 1细胞
BayGenomics ES cell line RRD031 Cells(提供STR鉴定图谱)
BayGenomics ES cell line XE767 Cells(提供STR鉴定图谱)
DA-3/TM Cells(提供STR鉴定图谱)
MIN6 Cells(提供STR鉴定图谱)
Swiss5 Cells(提供STR鉴定图谱)
LTC-14 Cells(提供STR鉴定图谱)
" "PubMed=4894370; DOI=10.1002/1097-0142(196908)24:2<211::AID-CNCR2820240202>3.0.CO;2-3
Southam C.M., Burchenal J.H., Clarkson B.D. Sr., Tanzi A., Mackey R., McComb V.
Heterotransplantability of human cell lines derived from leukemia and lymphomas into immunologically tolerant rats.
Cancer 24:211-222(1969)
DOI=10.1007/BF02618370
Stulberg C.S., Coriell L.L., Kniazeff A.J., Shannon J.E.
The animal cell culture collection.
In Vitro 5:1-16(1970)
PubMed=4321017; DOI=10.1002/ijc.2910060315
Durr F.E., Monroe J.H., Schmitter R., Traul K.A., Hirshaut Y.
Studies on the infectivity and cytopathology of Epstein-Barr virus in human lymphoblastoid cells.
Int. J. Cancer 6:436-449(1970)
PubMed=4321974
Maurer B.A., Imamura T., Wilbert S.M.
Incidence of EB virus-containing cells in primary and secondary clones of several Burkitt lymphoma cell lines.
Cancer Res. 30:2870-2875(1970)
PubMed=4325933; DOI=10.1093/jnci/46.6.1243
Pearson G.R., Henle G.S., Henle W.
Production of antigens associated with Epstein-Barr virus in experimentally infected lymphoblastoid cell lines.
J. Natl. Cancer Inst. 46:1243-1250(1971)
PubMed=4122458; DOI=10.1002/ijc.2910100108
Klein G., Dombos L., Gothoskar B.
Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus.
Int. J. Cancer 10:44-57(1972)
PubMed=4347031; DOI=10.1093/jnci/48.1.87
Hewetson J.F., Gothoskar B., Klein G.
Radioiodine-labeled antibody test for the detection of membrane antigens associated with Epstein-Barr virus.
J. Natl. Cancer Inst. 48:87-94(1972)
PubMed=4550511; DOI=10.1073/pnas.69.1.78; PMCID=PMC427548
Hampar B., Derge J.G., Martos L.M., Walker J.L.
Synthesis of Epstein-Barr virus after activation of the viral genome in a 'virus-negative' human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine.
Proc. Natl. Acad. Sci. U.S.A. 69:78-82(1972)
PubMed=4364259; DOI=10.1002/ijc.2910110210
Klein G., Dombos L.
Relationship between the sensitivity of EBV-carrying lymphoblastoid lines to superinfection and the inducibility of the resident viral genome.
Int. J. Cancer 11:327-337(1973)
PubMed=4736620; DOI=10.1111/j.1469-1809.1973.tb00588.x
Povey S., Gardiner S.E., Watson B., Mowbray S., Harris H., Arthur E., Steel C.M., Blenkinsop C., Evans H.J.
Genetic studies on human lymphoblastoid lines: isozyme analysis on cell lines from forty-one different individuals and on mutants produced following exposure to a chemical mutagen.
Ann. Hum. Genet. 36:247-266(1973)
PubMed=4366935
Minowada J., Nonoyama M., Moore G.E., Rauch A.M., Pagano J.S.
The presence of the Epstein-Barr viral genome in human lymphoblastoid B-cell lines and its absence in a myeloma cell line.
Cancer Res. 34:1898-1903(1974)
PubMed=168255; DOI=10.4049/jimmunol.115.1.243
Hutt L.M., Huang Y.-T., Dascomb H.E., Pagano J.S.
Enhanced destruction of lymphoid cell lines by peripheral blood leukocytes taken from patients with acute infectious mononucleosis.
J. Immunol. 115:243-248(1975)
PubMed=170370; DOI=10.1099/0022-1317-28-2-207
Adams A., Strander H., Cantell K.
Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon.
J. Gen. Virol. 28:207-217(1975)
PubMed=1086134
Kaplan J., Peterson W.D. Jr.
Detection of T-cell lymphoma-associated antigens on cord blood lymphocytes and phytohemagglutinin-stimulated blasts.
Cancer Res. 36:3471-3475(1976)
PubMed=216485
Higgins N.P., Strauss B.S.
Differences in the ability of human lymphoblastoid lines to exclude bromodeoxyuridine and in their sensitivity to methyl methanesulfonate and to incorporated [3H]thymidine.
Cancer Res. 39:312-320(1979)
PubMed=7316467; DOI=10.1111/j.1469-1809.1980.tb00953.x
Povey S., Jeremiah S., Arthur E., Steel M., Klein G.
Differences in genetic stability between human cell lines from patients with and without lymphoreticular malignancy.
Ann. Hum. Genet. 44:119-133(1980)
PubMed=6265077
Pizzo P.A., Chattopadhyay S.K., Magrath I.T., Del Giacco E., Sherrick D., Gray T.E.
Examination of Epstein-Barr virus and C-type proviral sequences in American and African lymphomas and derivative cell lines.
Cancer Res. 41:3165-3171(1981)
PubMed=6286763; DOI=10.4049/jimmunol.129.3.1336
Benjamin D., Magrath I.T., Maguire R.T., Janus C., Todd-Kulikowsk H.D., Parsons R.G.
Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt's and non-Burkitt's type.
J. Immunol. 129:1336-1342(1982)
PubMed=6806672; DOI=10.1038/298474a0
Lenoir G.M., Preud'homme J.-L., Bernheim A., Berger R.
Correlation between immunoglobulin light chain expression and variant translocation in Burkitt's lymphoma.
Nature 298:474-476(1982)
PubMed=6954533; DOI=10.1073/pnas.79.7.2194; PMCID=PMC346157
Westin E.H., Gallo R.C., Arya S.K., Eva A., Souza L.M., Baluda M.A., Aaronson S.A., Wong-Staal F.
Differential expression of the amv gene in human hematopoietic cells.
Proc. Natl. Acad. Sci. U.S.A. 79:2194-2198(1982)
PubMed=7060222; DOI=10.1016/0009-2797(82)90007-2
Meltz M.L., Whittam N.J., Thornburg W.H.
Reassociation of human lymphoblastoid cell DNA repair replicated following methyl methanesulfonate treatment.
Chem. Biol. Interact. 39:77-88(1982)
PubMed=6306472; DOI=10.1038/304135a0
Hamlyn P.H., Rabbitts T.H.
Translocation joins c-myc and immunoglobulin gamma 1 genes in a Burkitt lymphoma revealing a third exon in the c-myc oncogene.
Nature 304:135-139(1983)
PubMed=6419122; DOI=10.1038/306760a0
Rabbitts T.H., Hamlyn P.H., Baer R.
Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma.
Nature 306:760-765(1983)
PubMed=6600440; DOI=10.1007/BF02617996
Uittenbogaart C.H., Cantor Y., Fahey J.L.
Growth of human malignant lymphoid cell lines in serum-free medium.
In Vitro 19:67-72(1983)
PubMed=6231253; DOI=10.1002/ijc.2910330407
Ehlin-Henriksson B., Klein G.
Distinction between Burkitt lymphoma subgroups by monoclonal antibodies: relationships between antigen expression and type of chromosomal translocation.
Int. J. Cancer 33:459-463(1984)
PubMed=6500159; DOI=10.1159/000163283
Gershwin M.E., Lentz D., Owens R.B.
Relationship between karyotype of tissue culture lines and tumorigenicity in nude mice.
Exp. Cell Biol. 52:361-370(1984)
PubMed=6547209; DOI=10.1038/309592a0
Rabbitts T.H., Forster A., Hamlyn P.H., Baer R.
Effect of somatic mutation within translocated c-myc genes in Burkitt's lymphoma.
Nature 309:592-597(1984)
PubMed=6582512; DOI=10.1073/pnas.81.2.568; PMCID=PMC344720
Mattes M.J., Cordon-Cardo C., Lewis J.L. Jr., Old L.J., Lloyd K.O.
Cell surface antigens of human ovarian and endometrial carcinoma defined by mouse monoclonal antibodies.
Proc. Natl. Acad. Sci. U.S.A. 81:568-572(1984)
PubMed=6592381; DOI=10.1093/jnci/73.4.841
Favrot M.-C., Philip I., Philip T., Portoukalian J., Dore J.-F., Lenoir G.M.
Distinct reactivity of Burkitt's lymphoma cell lines with eight monoclonal antibodies correlated with the ethnic origin.
J. Natl. Cancer Inst. 73:841-847(1984)
PubMed=2985879; DOI=10.1016/0145-2126(85)90084-0
Drexler H.G., Gaedicke G., Minowada J.
Isoenzyme studies in human leukemia-lymphoma cell lines -- 1 carboxylic esterase.
Leuk. Res. 9:209-229(1985)
PubMed=2998993
Steel C.M., Morten J.E.N., Foster E.
The cytogenetics of human B lymphoid malignancy: studies in Burkitt's lymphoma and Epstein-Barr virus-transformed lymphoblastoid cell lines.
IARC Sci. Publ. 60:265-292(1985)
PubMed=3159941; DOI=10.1016/0145-2126(85)90134-1
Drexler H.G., Gaedicke G., Minowada J.
Isoenzyme studies in human leukemia-lymphoma cell lines -- III Beta-hexosaminidase (E.C. 3.2.1.30).
Leuk. Res. 9:549-559(1985)
PubMed=3874327; DOI=10.1016/0145-2126(85)90133-x
Drexler H.G., Gaedicke G., Minowada J.
Isoenzyme studies in human leukemia-lymphoma cells lines -- II. Acid phosphatase.
Leuk. Res. 9:537-548(1985)
PubMed=3905596
Favrot M.-C., Philip I., Philip T., Cabrillat H., Pinatel C., Dore J.-F., Lenoir G.M.
Immunophenotypic classification of 28 Burkitt cell lines with monoclonal antibodies and reagent selection for bone-marrow purging.
IARC Sci. Publ. 60:447-452(1985)
PubMed=3080238
Sieverts H., Alabaster O., Goldschmidts W., Magrath I.T.
Expression of surface antigens during the cell cycle in different growth phases of American and African Burkitt's lymphoma cell lines.
Cancer Res. 46:1182-1188(1986)
PubMed=3100061; DOI=10.1016/0008-8749(86)90099-7
Benjamin D., Bazar L.S., Wallace B., Jacobson R.J.
Heterogeneity of B-cell growth factor receptor reactivity in healthy donors and in patients with chronic lymphatic leukemia: relationship to B-cell-derived lymphokines.
Cell. Immunol. 103:394-408(1986)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=3026973; DOI=10.1002/ijc.2910390215
Ehlin-Henriksson B., Manneborg-Sandlund A., Klein G.
Expression of B-cell-specific markers in different Burkitt lymphoma subgroups.
Int. J. Cancer 39:211-218(1987)
PubMed=3034807; DOI=10.1002/ijc.2910390622
Ohno H., Fukuhara S., Takahashi R., Mihara K.-i., Sugiyama T., Doi S., Uchino H., Toyoshima K.
c-yes and bcl-2 genes located on 18q21.3 in a follicular lymphoma cell line carrying a t(14;18) chromosomal translocation.
Int. J. Cancer 39:785-788(1987)
PubMed=2470097; DOI=10.1073/pnas.86.9.3257; PMCID=PMC287109
Shtivelman E., Henglein B., Groitl P., Lipp M., Bishop J.M.
Identification of a human transcription unit affected by the variant chromosomal translocations 2;8 and 8;22 of Burkitt lymphoma.
Proc. Natl. Acad. Sci. U.S.A. 86:3257-3260(1989)
PubMed=2140233; DOI=10.1111/j.1440-1827.1990.tb01549.x
Nakano A., Harada T., Morikawa S., Kato Y.
Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines.
Acta Pathol. Jpn. 40:107-115(1990)
PubMed=1915267; DOI=10.1002/j.1460-2075.1991.tb07837.x; PMCID=PMC452998
Farrell P.J., Allan G.J., Shanahan F., Vousden K.H., Crook T.
p53 is frequently mutated in Burkitt's lymphoma cell lines.
EMBO J. 10:2879-2887(1991)
CLPUB00447
Mulivor R.A., Suchy S.F.
1992/1993 catalog of cell lines. NIGMS human genetic mutant cell repository. 16th edition. October 1992.
(In misc. document) Institute for Medical Research (Camden, N.J.) NIH 92-2011; pp.1-918; National Institutes of Health; Bethesda; USA (1992)
PubMed=1325212; DOI=10.1182/blood.V80.5.1289.1289
Benjamin D., Knobloch T.J., Dayton M.A.
Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt's lymphoma constitutively secrete large quantities of interleukin-10.
Blood 80:1289-1298(1992)
CLPUB00458
Treichel R.S.
Susceptibility to LAK-mediated cytotoxicity of multidrug-resistant variants of the human RAJI cell line is not related to expression of major cellular adhesion molecules.
Ohio J. Sci. 93:14-18(1993)
PubMed=8316623; DOI=10.2307/3578190
Evans H.H., Ricanati M., Horng M.-F., Jiang Q.-Y., Mencl J., Olive P.L.
DNA double-strand break rejoining deficiency in TK6 and other human B-lymphoblast cell lines.
Radiat. Res. 134:307-315(1993)
PubMed=8344493; DOI=10.1096/fasebj.7.10.8344493
Bhatia K.G., Goldschmidts W., Gutierrez M.I., Gaidano G., Dalla-Favera R., Magrath I.T.
Hemi- or homozygosity: a requirement for some but not other p53 mutant proteins to accumulate and exert a pathogenetic effect.
FASEB J. 7:951-956(1993)
PubMed=8515068; DOI=10.4049/jimmunol.150.12.5418
Jain V.K., Judde J.-G., Max E.E., Magrath I.T.
Variable IgH chain enhancer activity in Burkitt's lymphomas suggests an additional, direct mechanism of c-myc deregulation.
J. Immunol. 150:5418-5428(1993)
PubMed=8176200; DOI=10.4049/jimmunol.152.10.4749
Benjamin D., Sharma V., Knobloch T.J., Armitage R.J., Dayton M.A., Goodwin R.G.
B cell IL-7. Human B cell lines constitutively secrete IL-7 and express IL-7 receptors.
J. Immunol. 152:4749-4757(1994)
PubMed=7849311; DOI=10.1182/blood.V85.4.893.bloodjournal854893
Stranks G., Height S.E., Mitchell P., Jadayel D.M., Yuille M.A.R., De Lord C.F.M., Clutterbuck R.D., Treleaven J.G., Powles R.L., Nacheva E., Oscier D.G., Karpas A., Lenoir G.M., Smith S.D., Millar J.L., Catovsky D., Dyer M.J.S.
Deletions and rearrangement of CDKN2 in lymphoid malignancy.
Blood 85:893-901(1995)
PubMed=8547074; DOI=10.1111/j.1365-2141.1995.tb05302.x
Siebert R., Willers C.P., Schramm A., Fossa A., Dresen I.M.G., Uppenkamp M.J., Nowrousian M.R., Seeber S., Opalka B.
Homozygous loss of the MTS1/p16 and MTS2/p15 genes in lymphoma and lymphoblastic leukaemia cell lines.
Br. J. Haematol. 91:350-354(1995)
PubMed=8558913
Morita S., Tsuchiya S., Fujie H., Itano M., Ohashi Y., Minegishi M., Imaizumi M., Endo M., Takano N., Konno T.
Cell surface c-kit receptors in human leukemia cell lines and pediatric leukemia: selective preservation of c-kit expression on megakaryoblastic cell lines during adaptation to in vitro culture.
Leukemia 10:102-105(1996)
PubMed=8568269; DOI=10.4049/jimmunol.156.4.1626
Benjamin D., Sharma V., Kubin M., Klein J.L., Sartori A., Holliday J., Trinchieri G.
IL-12 expression in AIDS-related lymphoma B cell lines.
J. Immunol. 156:1626-1637(1996)
PubMed=8847894
Tani A., Tatsumi E., Nakamura F., Kumagai S., Kosaka Y., Sano K., Nakamura H., Amakawa R., Ohno H.
Sensitivity to dexamethasone and absence of bcl-2 protein in Burkitt's lymphoma cell line (Black93) derived from a patient with acute tumor lysis syndrome: comparative study with other BL and non-BL lines.
Leukemia 10:1592-1603(1996)
PubMed=9192833
Cherney B.W., Bhatia K.G., Sgadari C., Gutierrez M.I., Mostowski H.S., Pike S.E., Gupta G., Magrath I.T., Tosato G.
Role of the p53 tumor suppressor gene in the tumorigenicity of Burkitt's lymphoma cells.
Cancer Res. 57:2508-2515(1997)
PubMed=9473234; DOI=10.1182/blood.V91.5.1680
Klangby U., Okan I., Magnusson K.P., Wendland M., Lind P., Wiman K.G.
p16/INK4a and p15/INK4b gene methylation and absence of p16/INK4a mRNA and protein expression in Burkitt's lymphoma.
Blood 91:1680-1687(1998)
PubMed=9510473; DOI=10.1111/j.1349-7006.1998.tb00476.x; PMCID=PMC5921588
Hosoya N., Hangaishi A., Ogawa S., Miyagawa K., Mitani K., Yazaki Y., Hirai H.
Frameshift mutations of the hMSH6 gene in human leukemia cell lines.
Jpn. J. Cancer Res. 89:33-39(1998)
PubMed=9685479; DOI=10.1093/nar/26.16.3651; PMCID=PMC147775
Hultdin M., Gronlund E., Norrback K.-F., Eriksson-Lindstrom E., Just T., Roos G.
Telomere analysis by fluorescence in situ hybridization and flow cytometry.
Nucleic Acids Res. 26:3651-3656(1998)
PubMed=9737686; DOI=10.1038/sj.leu.2401112
Zhang W.-J., Ohnishi K., Shigeno K., Fujisawa S., Naito K., Nakamura S., Takeshita K., Takeshita A., Ohno R.
The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms.
Leukemia 12:1383-1391(1998)
PubMed=9738977; DOI=10.1111/j.1349-7006.1998.tb03275.x; PMCID=PMC5921886
Takizawa J., Suzuki R., Kuroda H., Utsunomiya A., Kagami Y., Joh T., Aizawa Y., Ueda R., Seto M.
Expression of the TCL1 gene at 14q32 in B-cell malignancies but not in adult T-cell leukemia.
Jpn. J. Cancer Res. 89:712-718(1998)
PubMed=9787181; DOI=10.1182/blood.V92.9.3410
Sakai A., Thieblemont C., Wellmann A., Jaffe E.S., Raffeld M.
PTEN gene alterations in lymphoid neoplasms.
Blood 92:3410-3415(1998)
PubMed=9973220
Gutierrez M.I., Cherney B.W., Hussain A., Mostowski H.S., Tosato G., Magrath I.T., Bhatia K.G.
Bax is frequently compromised in Burkitt's lymphomas with irreversible resistance to Fas-induced apoptosis.
Cancer Res. 59:696-703(1999)
PubMed=10739008; DOI=10.1016/S0145-2126(99)00182-4
Inoue K., Kohno T., Takakura S., Hayashi Y., Mizoguchi H., Yokota J.
Frequent microsatellite instability and BAX mutations in T cell acute lymphoblastic leukemia cell lines.
Leuk. Res. 24:255-262(2000)
PubMed=11226526; DOI=10.1016/S0145-2126(00)00121-1
Inoue K., Kohno T., Takakura S., Hayashi Y., Mizoguchi H., Yokota J.
Corrigendum to: Frequent microsatellite instability and BAX mutations in T cell acute lymphoblastic leukemia cell lines Leukemia Research 24 (2000), 255-262.
Leuk. Res. 25:275-278(2001)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=12145705; DOI=10.1038/sj.leu.2402519
Langerak A.W., Moreau E.J., van Gastel-Mol E.J., van der Burg M., van Dongen J.J.M.
Detection of clonal EBV episomes in lymphoproliferations as a diagnostic tool.
Leukemia 16:1572-1573(2002)
PubMed=12967475; DOI=10.1111/j.1349-7006.2003.tb01518.x; PMCID=PMC11160262
Maesako Y., Uchiyama T., Ohno H.
Comparison of gene expression profiles of lymphoma cell lines from transformed follicular lymphoma, Burkitt's lymphoma and de novo diffuse large B-cell lymphoma.
Cancer Sci. 94:774-781(2003)
PubMed=14982850; DOI=10.1016/S0002-9440(10)63184-7; PMCID=PMC1614712
Takakuwa T., Luo W.-J., Ham M.F., Sakane-Ishikawa E., Wada N., Aozasa K.
Integration of Epstein-Barr virus into chromosome 6q15 of Burkitt lymphoma cell line (Raji) induces loss of BACH2 expression.
Am. J. Pathol. 164:967-974(2004)
PubMed=15028022; DOI=10.1111/j.1440-1827.2004.01612.x
Kamimura K., Hojo H., Abe M.
Characterization of expression of protein kinase C isozymes in human B-cell lymphoma: relationship between its expression and prognosis.
Pathol. Int. 54:224-230(2004)
PubMed=15457187; DOI=10.1038/sj.leu.2403534
Karpova M.B., Schoumans J., Ernberg I., Henter J.-I., Nordenskjold M., Fadeel B.
Raji revisited: cytogenetics of the original Burkitt's lymphoma cell line.
Leukemia 19:159-161(2005)
PubMed=15901131; DOI=10.1016/j.prp.2005.01.002
Murai Y., Hayashi S., Takahashi H., Tsuneyama K., Takano Y.
Correlation between DNA alterations and p53 and p16 protein expression in cancer cell lines.
Pathol. Res. Pract. 201:109-115(2005)
PubMed=18357372; DOI=10.3892/or.19.4.889
Pop I., Pop L., Vitetta E.S., Ghetie M.-A.
Generation of multidrug resistant lymphoma cell lines stably expressing P-glycoprotein.
Oncol. Rep. 19:889-895(2008)
PubMed=19358282; DOI=10.1002/ijc.24351
Inagaki A., Ishida T., Yano H., Ishii T., Kusumoto S., Ito A., Ri M., Mori F., Ding J.-M., Komatsu H., Iida S., Ueda R.
Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC.
Int. J. Cancer 125:212-221(2009)
PubMed=20164919; DOI=10.1038/nature08768; PMCID=PMC3145113
Bignell G.R., Greenman C.D., Davies H.R., Butler A.P., Edkins S., Andrews J.M., Buck G., Chen L., Beare D., Latimer C., Widaa S., Hinton J., Fahey C., Fu B.-Y., Swamy S., Dalgliesh G.L., Teh B.T., Deloukas P., Yang F.-T., Campbell P.J., Futreal P.A., Stratton M.R.
Signatures of mutation and selection in the cancer genome.
Nature 463:893-898(2010)
PubMed=20215515; DOI=10.1158/0008-5472.CAN-09-3458; PMCID=PMC2881662
Rothenberg S.M., Mohapatra G., Rivera M.N., Winokur D., Greninger P., Nitta M., Sadow P.M., Sooriyakumar G., Brannigan B.W., Ulman M.J., Perera R.M., Wang R., Tam A., Ma X.-J., Erlander M., Sgroi D.C., Rocco J.W., Lingen M.W., Cohen E.E.W., Louis D.N., Settleman J., Haber D.A.
A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers.
Cancer Res. 70:2158-2164(2010)
PubMed=20454443; DOI=10.1155/2010/904767; PMCID=PMC2861168
Uphoff C.C., Denkmann S.A., Steube K.G., Drexler H.G.
Detection of EBV, HBV, HCV, HIV-1, HTLV-I and -II, and SMRV in human and other primate cell lines.
J. Biomed. Biotechnol. 2010:904767.1-904767.23(2010)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=22885699; DOI=10.1038/nature11378; PMCID=PMC3609867
Schmitz R., Young R.M., Ceribelli M., Jhavar S., Xiao W.-M., Zhang M.-L., Wright G., Shaffer A.L. 3rd, Hodson D.J., Buras E., Liu X.-L., Powell J.I., Yang Y.-D., Xu W.-H., Zhao H., Kohlhammer H., Rosenwald A., Kluin P.M., Muller-Hermelink H.-K., Ott G., Gascoyne R.D., Connors J.M., Rimsza L.M., Campo E., Jaffe E.S., Delabie J., Smeland E.B., Ogwang M.D., Reynolds S.J., Fisher R.I., Braziel R.M., Tubbs R.R., Cook J.R., Weisenburger D.D., Chan W.-C., Pittaluga S., Wilson W., Waldmann T.A., Rowe M., Mbulaiteye S.M., Rickinson A.B., Staudt L.M.
Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics.
Nature 490:116-120(2012)
PubMed=24590883; DOI=10.1002/gcc.22161
Murga Penas E.-M., Schilling G., Behrmann P., Klokow M., Vettorazzi E., Bokemeyer C., Dierlamm J.
Comprehensive cytogenetic and molecular cytogenetic analysis of 44 Burkitt lymphoma cell lines: secondary chromosomal changes characterization, karyotypic evolution, and comparison with primary samples.
Genes Chromosomes Cancer 53:497-515(2014)
PubMed=25960936; DOI=10.4161/21624011.2014.954893; PMCID=PMC4355981
Boegel S., Lower M., Bukur T., Sahin U., Castle J.C.
A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines.
OncoImmunology 3:e954893.1-e954893.12(2014)
PubMed=25355872; DOI=10.1128/JVI.02570-14; PMCID=PMC4301145
Cao S.-B., Strong M.J., Wang X., Moss W.N., Concha M., Lin Z., O'Grady T., Baddoo M., Fewell C., Renne R., Flemington E.K.
High-throughput RNA sequencing-based virome analysis of 50 lymphoma cell lines from the Cancer Cell Line Encyclopedia project.
J. Virol. 89:713-729(2015)
PubMed=25485619; DOI=10.1038/nbt.3080
Klijn C., Durinck S., Stawiski E.W., Haverty P.M., Jiang Z.-S., Liu H.-B., Degenhardt J., Mayba O., Gnad F., Liu J.-F., Pau G., Reeder J., Cao Y., Mukhyala K., Selvaraj S.K., Yu M.-M., Zynda G.J., Brauer M.J., Wu T.D., Gentleman R.C., Manning G., Yauch R.L., Bourgon R., Stokoe D., Modrusan Z., Neve R.M., de Sauvage F.J., Settleman J., Seshagiri S., Zhang Z.-M.
A comprehensive transcriptional portrait of human cancer cell lines.
Nat. Biotechnol. 33:306-312(2015)
PubMed=25877200; DOI=10.1038/nature14397
Yu M., Selvaraj S.K., Liang-Chu M.M.Y., Aghajani S., Busse M., Yuan J., Lee G., Peale F.V., Klijn C., Bourgon R., Kaminker J.S., Neve R.M.
A resource for cell line authentication, annotation and quality control.
Nature 520:307-311(2015)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27566572; DOI=10.18632/oncotarget.11524; PMCID=PMC5325377
Quentmeier H., Pommerenke C., Ammerpohl O., Geffers R., Hauer V., MacLeod R.A.F., Nagel S., Romani J., Rosati E., Rosen A., Uphoff C.C., Zaborski M., Drexler H.G.
Subclones in B-lymphoma cell lines: isogenic models for the study of gene regulation.
Oncotarget 7:63456-63465(2016)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29892436; DOI=10.1098/rsos.172472; PMCID=PMC5990783
Shioda S., Kasai F., Watanabe K., Kawakami K., Ohtani A., Iemura M., Ozawa M., Arakawa A., Hirayama N., Kawaguchi E., Tano T., Miyata S., Satoh M., Shimizu N., Kohara A.
Screening for 15 pathogenic viruses in human cell lines registered at the JCRB Cell Bank: characterization of in vitro human cells by viral infection.
R. Soc. Open Sci. 5:172472-172472(2018)
PubMed=30285677; DOI=10.1186/s12885-018-4840-5; PMCID=PMC6167786
Tan K.-T., Ding L.-W., Sun Q.-Y., Lao Z.-T., Chien W., Ren X., Xiao J.-F., Loh X.-Y., Xu L., Lill M., Mayakonda A., Lin D.-C., Yang H.H., Koeffler H.P.
Profiling the B/T cell receptor repertoire of lymphocyte derived cell lines.
BMC Cancer 18:940.1-940.13(2018)
PubMed=30629668; DOI=10.1371/journal.pone.0210404; PMCID=PMC6328144
Uphoff C.C., Pommerenke C., Denkmann S.A., Drexler H.G.
Screening human cell lines for viral infections applying RNA-Seq data analysis.
PLoS ONE 14:E0210404-E0210404(2019)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)"