GMP Human FGF-8b Protein
优势特色(Features)
Designed under ISO 9001:2015 and ISO 13485:2016
Manufactured and QC tested under a GMP compliance factory
FDA DMF filed
Animal-Free materials
Beta-lactam materials free
Batch-to-batch consistency
Stringent quality control tests
No animal derived peptone and lactose used in production process
表达区间及表达系统(Source)
GMP Human FGF-8b Protein (GMP-FGBH16) is expressed from E. coli cells. It contains AA Gln 23 - Arg 215 (Accession # P55075-3).
蛋白结构(Molecular Characterization)

This protein carries no "tag".
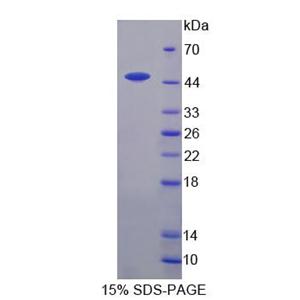
The protein has a calculated MW of 22.5 kDa. The protein migrates as 25 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE).
N端测序(N-terminal Sequence Analysis)
Met-Gln-Val-Thr-Val-Gln-Ser-Ser-Pro-Asn-Phe-Thr-Gln-His-Val (Routinely tested).
内毒素(Endotoxin)
Less than 10 EU/mg by the LAL method / rFC method.
宿主蛋白残留(Host Cell Protein)
0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
The sterility testing was performed by membrane filtration method described in USP<71>and Ph. Eur. 2.6.1.
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with blue ice, please inquire the shipping cost.
存储(Storage)
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
-20°C to -70°C for 5 years in lyophilized state;
-70°C for 12 months under sterile conditions after reconstitution.


背景介绍
FGF-8 is a member of the fibroblast growth factor family that was originally discovered as a growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells (1-3). Alternate splicing of mouse FGF-8 mR generates eight secreted isoforms, designated a-h, but only FGF-8a, b, e and f exist in humans (4). FGF-8 contains a 22 amino acid (aa) signal sequence, an N�terminal domain that varies according to the isoform (30 aa for FGF-8b; 20 aa for the shortest, FGF-8a), a 125 aa FGF domain and a 37 aa proline�rich C�terminal sequence. The FGF domain of FGF-8 shares the most aa identity with FGF17 (75%) and FGF-18 (67%), and the three form an FGF subfamily (2). Mouse FGF-8b shares 100% aa identity with human FGF-8b. FGF-8 is widely expressed during embryogenesis, and mediates epithelial-mesenchymal transitions. It plays an organizing and inducing role during gastrulation, and regulates patterning of the midbrain/hindbrain, eye, ear, limbs and heart in the embryo (2, 5 - 8). The isoforms may play different roles in development. FGF-8b shows the strongest receptor affinity and oncogenic transforming capacity although FGF-8a and FGF-8e are also transforming and have been found in human prostate, breast or ovarian tumors (1, 5, 9-12). FGF-8 shows limited expression in the normal adult, but low levels are found in the reproductive and genitourinary tract, peripheral leukocytes and bone marrow hematopoietic cells (3, 9, 13).
ACRO GMP产品制造规范
ACROBiosystems GMP级产品是在质量管理体系下生产的,并符合相关指南:
Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP<92>Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP<1043>Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems质量管理体系内容:
1. 根据ISO 9001:2015和ISO 13485:2016进行设计,在GMP工厂进行制造和QC检测
2. 无动物成分
3. QA从批准的供应商处采购的材料
4. ISO 5洁净室和自动灌装设备
5. 人员合格
6. 质量保证审核和批准质量相关文件
7. 全批量生产和控制记录
8. 设备维护和校准
9. 分析程序的验证
10. 进行的稳定性研究
11. 全面的法规支持文件
ACROBiosystems对我们的GMP级产品提供严格的质量控制测试(经过充分验证的设备、工艺和测试方法),以确保它们在纯度、安全性、活性和批间稳定性方面符合严格的标准,每个批量QC批次主要包含以下具体信息:
1. SDS-PAGE
2. 蛋白质含量
3. 内毒素水平
4. 残留宿主细胞DNA含量
5. 残留宿主细胞蛋白质含量
6. 生物活性分析
7. 微生物检测
8. 支原体检测
9. 体外病毒测定
10. 残留水分
11. 批次间一致性
ACRO产品声明
ACROBiosystems GMP级产品专为研究、生产或离体使用而设计。注意:不可直接供人体使用。