GMP Human SCF Protein
优势特色(Features)
Designed under ISO 9001:2015 and ISO 13485:2016
Manufactured and QC tested under a GMP compliance factory
Animal-Free materials
Beta-lactam materials free
Batch-to-batch consistency
Stringent quality control tests
表达区间及表达系统(Source)
GMP Human SCF Protein (GMP-SCFH25) is expressed from human 293 cells (HEK293). It contains AA Glu 26 - Ala 189 (Accession # P21583-1).
Predicted N-terminus: Glu 26
蛋白结构(Molecular Characterization)

This protein carries no "tag".
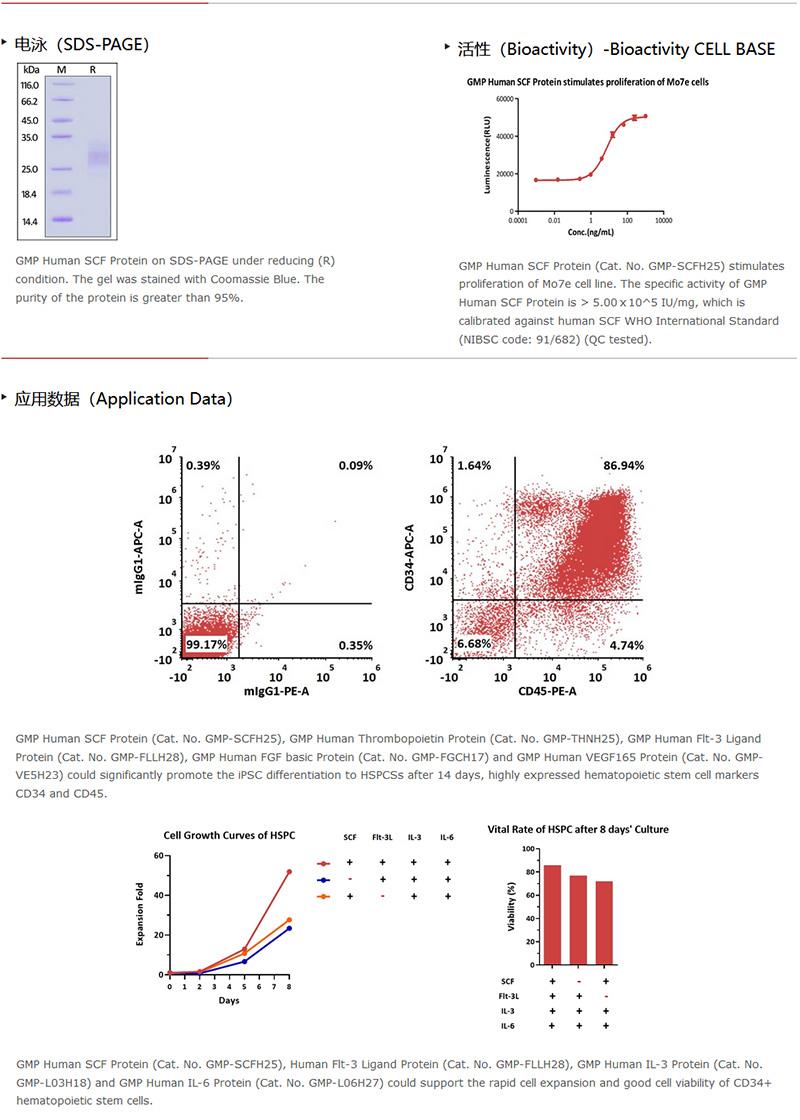
The protein has a calculated MW of 18.5 kDa. The protein migrates as 30 kDa±3 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 10 EU/mg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
The sterility testing was performed by membrane filtration method described in CP<1101>, USP<71> and Eur. Ph. 2.6.1.
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with blue ice, please inquire the shipping cost.
存储(Storage)
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
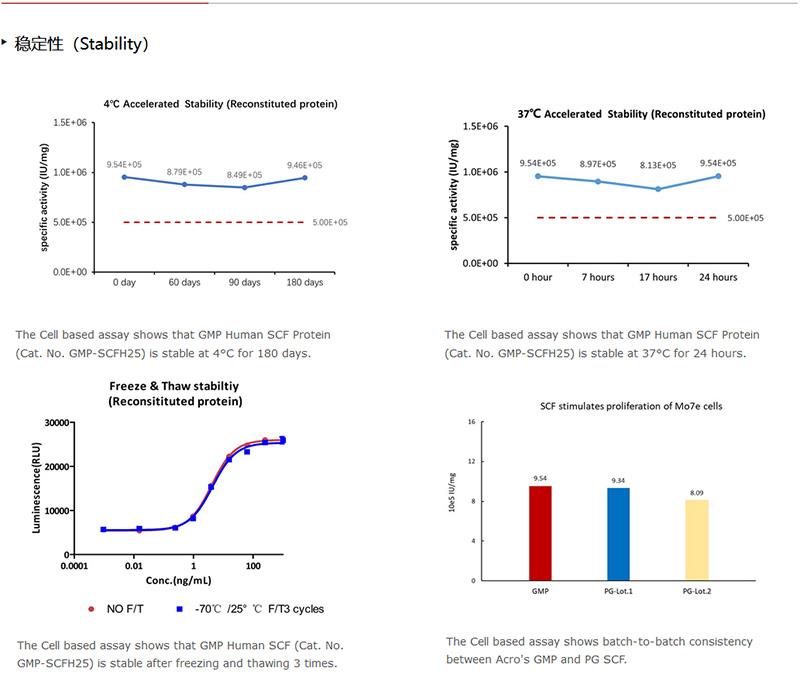
This product is stable after storage at:
-20°C to -70°C for 5 years in lyophilized state;
-70°C for 12 months under sterile conditions after reconstitution.
ACRO GMP产品制造规范
ACROBiosystems GMP级产品是在质量管理体系下生产的,并符合相关指南:
Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems质量管理体系内容:
1. 根据ISO 9001:2015和ISO 13485:2016进行设计,在GMP工厂进行制造和QC检测
2. 无动物成分
3. QA从批准的供应商处采购的材料
4. ISO 5洁净室和自动灌装设备
5. 人员合格
6. 质量保证审核和批准质量相关文件
7. 全批量生产和控制记录
8. 设备维护和校准
9. 分析程序的验证
10. 进行的稳定性研究
11. 全面的法规支持文件
ACROBiosystems对我们的GMP级产品提供严格的质量控制测试(经过充分验证的设备、工艺和测试方法),以确保它们在纯度、安全性、活性和批间稳定性方面符合严格的标准,每个批量QC批次主要包含以下具体信息:
1. SDS-PAGE
2. 蛋白质含量
3. 内毒素水平
4. 残留宿主细胞DNA含量
5. 残留宿主细胞蛋白质含量
6. 生物活性分析
7. 微生物检测
8. 支原体检测
9. 体外病毒测定
10. 残留水分
11. 批次间一致性
ACRO产品声明
ACROBiosystems GMP级产品专为研究、生产或离体使用而设计。注意:不可直接供人体使用。
背景(Background)
干细胞因子也称为SCF、试剂盒配体、KL、钢因子、KITLG、FPH2、KL-1、Kitl、MGF、SCF、SF或SHEP7,是一种与c-kit受体(CD117)结合的细胞因子。SCF既可以作为跨膜蛋白存在,也可以作为可溶性蛋白存在。这种细胞因子在造血(血细胞的形成)、精子发生和黑色素生成中起着重要作用。该蛋白的可溶性和跨膜形式是通过同一R转录物的选择性剪接形成的。可溶性和跨膜SCF由成纤维细胞和内皮细胞产生。可溶性SCF的分子量为18.5 KDa,形成二聚体。SCF在胚胎发育过程中的造血过程中起着重要作用。造血发生的部位,如胎儿肝脏和骨髓,都表达SCF。在发育过程中,SCF的存在在黑素细胞的定位中也起着重要作用,黑素细胞是产生黑色素和控制色素沉着的细胞。SCF在骨髓干细胞生态位中对HSC的调节中起着重要作用。SCF可以与其他细胞因子一起用于培养HSC和造血祖细胞。这些细胞在体外(体外)的扩增将使骨髓移植取得进展,在骨髓移植中,造血干细胞被转移到患者体内以重建血液形成。