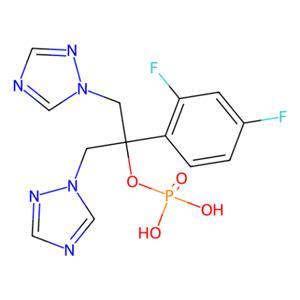
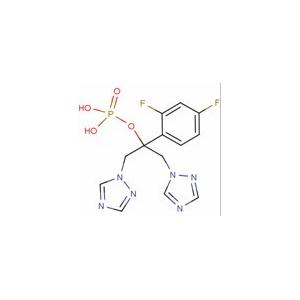
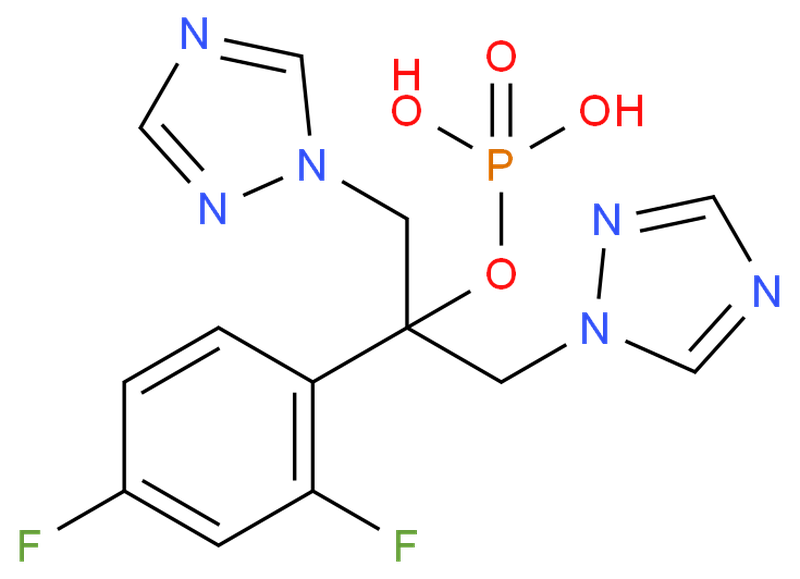
| 名称 | Fosfluconazole |
| 描述 | Fosfluconazole is water-soluble phosphate prodrug of fluconazole. Fluconazole is an antifungal drug. |
| 激酶实验 | An aliquot of 200 μl of mucosa scrap lysate solution was mixed with 100 mM phosphate buffer, pH 7.4, to a final volume at 1 ml. The concentration of the test compounds (fosphenytoin and fosfluconazole) was 10 μM. The incubation medium was prewarmed at 37°C before the reaction was initiated by addition of the tested compounds. An aliquot of 100 μl was collected from the incubation vial at the time points 0, 5, 10, 20, 30, 45, and 60 min and transferred to a 96-well plate, in which 100 μl of acetonitrile was prefilled to terminate the reaction. The samples were diluted 5-fold with acetonitrile containing 1 μM tolbutamide as an analytical internal standard. The samples were centrifuged at 4000 rpm for 5 min to precipitate protein. The supernatant was transferred to a new 96-well plate for concentration analysis by liquid chromatography/tandem mass spectrometry (LC/MS/MS) [1]. |
| 动物实验 | Twelve-week-old male Wistar rats (200–300 g) were used. They were housed in a temperature-controlled room and were given food and water ad libitum. All rats were anesthetized by an i.p. administration of pentobarbital (50 mg/kg body weight). A catheter was placed in the peritoneal cavity and used as an inflow drain for the dialyzing fluid. sampling. In rats receiving i.v. administration, a catheter was inserted into the left jugular vein for drug administration, and another into the right femoral artery for blood sampling. Blood samples after the i.p. administration were obtained from the heart. The rats were then allowed to recover from the anesthesia and surgery for at least 24 hr. After the 40-mL dialyzing fluid was administered intraperitoneally, FLCZ 16 mg/kg body weight) and F-FLCZ (16 mg FLCZ eq/kg body weight) were administered intravenously. The volume of the drug solution administered intravenously was 8 mL/kg. FLCZ (16 mg/kg body weight) and F-FLCZ (16 mg FLCZ eq/kg body weight) dissolved in the 40 mL of dialyzing fluid were administered intraperitoneally. The dialyzing fluid FLCZ concentration was 100 mg/L. F-FLCZ (16 mg FLCZ eq/kg body weight) was administered to ARF rats intravenously and intraperitoneally. Blood (0.5 mL) and dialyzing ‰uid (1.5 mL) samples were collected at appropriate time intervals [2]. |
| 体外活性 | 在Caco-2单层细胞中,10 μM Fosfluconazole在Transwell板的顶端或底部隔室中给药。两种前药在顶端隔室中经过2小时孵化后被有效裂解。ALP介导的转化率随前药浓度呈依赖性,以Michaelis-Menten常数为标准,Fosfluconazole在Caco-2细胞中的测定值为351μM[1]。 |
| 体内活性 | Fosfluconazole通过静脉和腹膜内给药。在腹膜内给予F-FLCZ后,FLCZ在循环血液和腹膜透析大鼠的透析液中被检测到。腹膜内给药后血浆中FLCZ的浓度低于静脉给药(F-FLCZ)后的浓度[2]。 |
| 存储条件 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| 溶解度 | DMSO : 6 mg/mL (15.53 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 1 mg/mL (2.59 mM), Sonication is recommended.
|
| 关键字 | Inhibitor | inhibit | Fungal | Fosfluconazole | Antifungal |
| 相关产品 | D-Gluconic acid (solution) (50% in H2O) | Calcium Propionate | Benzyl propionate | Potassium gluconate | Dehydroacetic acid sodium | Geraniol | Sulfacetamide sodium | octanal | 2-Methylcyclohexanone | L-Citronellol | Chitin | Sodium formate |
| 相关库 | 经典已知活性库 | 抗真菌库 | 已知活性化合物库 | 肝脏毒性化合物库 | ReFRAME 相关化合物库 | 抗感染化合物库 | FDA上市及药典收录分子库 | 上市药物库 | FDA 上市药物库 | 免疫/炎症分子化合物库 | 药物功能重定位化合物库 | 毒性化合物库 |