NOMENCLATURE
pyrethrins (pyrethrum)
Common name pyrethrins (BSI, E-ISO, ESA, JMAF); pyrhres (F-ISO)
CAS RN [8003-34-7]
pyrethrins (chrysanthemates)
Common name pyrethrin I (BSI, E-ISO); jasmoline I (F-ISO); accepted in lieu of a common name
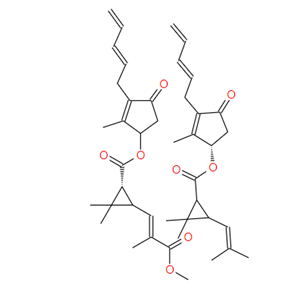
IUPAC name for pyrethrin I, Roth: (Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (+)-trans-chrysanthemate;
for cinerin I, Roth: (Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl (+)-trans-chrysanthemate;
for jasmolin I, Roth: (Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl (+)-trans-chrysanthemate
Chemical Abstracts name for pyrethrin I, [1R-[1a[S*(Z)],3b]]-2-methyl-4-oxo-3-(2,4-pentadienyl)cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate;
for cinerin I, [1R-[1a[S*(Z)],3b]]-3-(2-butenyl)-2-methyl-4-oxo-2-cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
; for jasmolin I, [1R-[1a[S*(Z)],3b]]-2-methyl-4-oxo-3-(2-pentenyl)-2-cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
CAS RN [121-21-1] pyrethrin I; [25402-06-6] cinerin I; [4466-14-2] jasmolin I EEC no. 204-455-8 (pyrethrin I); 246-948-0 (cinerin I)
pyrethrins (pyrethrates)
Common name pyrethrin II (BSI, E-ISO); cinerin II (BSI, E-ISO); jasmolin II (BSI, E-ISO); jasmoline II (F-ISO); accepted in lieu of a common name
IUPAC name for pyrethrin II, Roth: (Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (E)-(1R)-trans-3-(2-methoxycarbonylprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl pyrethrate
; for cinerin II, Roth: (Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl (E)-(1R)-trans-3-(2-methoxycarbonylprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate; or
(Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl pyrethrate
; for jasmolin II, Roth: (Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl (E)-(1R)-trans-3-(2-methoxycarbonylprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl pyrethrate
Chemical Abstracts name for pyrethrin II, [1R-[1a[S*(Z)],3b(E)]]-2-methyl-4-oxo-3-(2,4-pentadienyl)-2-cyclopenten-1-yl 3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2,2-dimethylcyclopropanecarboxylate;
for cinerin II, [1R-[1a[S*(Z)],3b(E)]]-3-(2-butenyl)-2-methyl-4-oxo-2-cyclopenten-1-yl 3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2,2-dimethylcyclopropanecarboxylate;
for jasmolin II, [1R-[1a[S*(Z)],3b(E)]]-2-methyl-4-oxo-3-(2-pentenyl)-2-cyclopent-1-enyl 3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2,2-dimethylcyclopropanecarboxylate
CAS RN [121-29-9] pyrethrin II; [121-20-0] cinerin II; [1172-63-0] jasmolin II EEC no. 204-462-6 (pyrethrin II); 204-454-2 (cinerin II)
PHYSICAL CHEMISTRY
pyrethrins (pyrethrum)
Composition Pyrethrum extract is defined as a mixture of three naturally-occurring, closely-related insecticidal esters of chrysanthemic acid, Pyrethrins I, and the three corresponding esters of pyrethrin acid, Pyrethrins II. In the USA, it is standardised as 50?% w/w total pyrethrins, but samples may be 20%; the ratio of Pyrethrins I to II is typically in the range 0.8-2.8:1; the ratio of individual esters (pyrethrins:cinerins:jasmolins) is 71:21:7. In Europe, pyrethrum extract of 25?.5% pyrethrins is common, with a 50?% formulation becoming more popular. The three components of Pyrethrins I (all with R = CH3) are pyrethrin I (R1 = CH=CH2); cinerin I (R1 = CH3); jasmolin I (R1 = CH2CH3); the components of Pyrethrins II (R = CO2CH3) correspond. Form Refined extract is a pale yellow, mobile oil, with a faint flowery odour; unrefined extract is a dark greenish-brown, viscous liquid. Powder (ground flowers) is tan colour. S.g./density 0.84-0.86 (25% pale extract), c. 0.9 (oleoresin crude) Solubility Pyrethrins are soluble in organic solvents, e.g. alcohols, hydrocarbons, aromatics, esters, etc. Stability Stable >10 y in absence of light, at ambient temperature. In light, rapid oxidation and inactivation occurs; DT50 in sunlight 10-12 min. Also destroyed by alkali, and by clay.
pyrethrins (chrysanthemates)
Mol. wt. pyrethrin I: 328.4; cinerin I: 316.4; jasmolin I: 330.5 M.f. pyrethrin I: C21H28O3; cinerin I: C20H28O3; jasmolin I: C21H30O3 B.p. 170 篊/0.1 mmHg (pyrethrin I) V.p. 2.7 mPa (pyrethrin I) KOW logP = 5.9 (pyrethrin I) Solubility In water 0.2 ppm (pyrethrin I). Specific rotation [a]D20 -14?(iso-octane) (pyrethrin I)
pyrethrins (pyrethrates)
Mol. wt. pyrethrin II: 372.4; cinerin II: 360.4; jasmolin II: 374.5 M.f. pyrethrin II: C22H28O5; cinerin II: C21H28O5; jasmolin II: C22H30O5 B.p. 200 /0.1 mmHg (pyrethrin II) V.p. 5.3 ?10-2 mPa (pyrethrin II) KOW logP = 4.3 (pyrethrin II) Solubility In water 9.0 ppm (pyrethrin II). Specific rotation [a]D20 +14.7?(iso-octane) (pyrethrin II)
COMMERCIALISATION
Production Pyrethrum is extracted from the flower Tanacetum (= Chrysanthemum = Pyrethrum)cinerariaefolium. Theextract is refined using methanol (Pyrethrum Board of Kenya and MGK) or carbon dioxide (Botanical Resources, Agropharm). History Pyrethrum was identified in antiquity in China. It spread west to Persia (Iran), probably via the Silk Roads during the Middle Ages. Dried powdered flower heads were known as "Persian Insect Powder". Records of use date from the early 19th century when it was introduced to Dalmatia, France, United States and Japan. Current production is mainly from East Africa (1930) and Australia (1980). Manufacturers Agrowonderful; Botanical Resources; MGK; Pyrethrum Board of Kenya
APPLICATIONS
Biochemistry Binds to sodium channels, prolonging their opening and thereby causing knockdown and death. Mode of action Non-systemic insecticide with contact action. Causes rapid knockdown (caused mainly by Pyrethrins II), with death occurring later (associated with Pyrethrins I). Has some acaricidal activity. Uses Control of a wide range of insects and mites in public health, stored products, animal houses, and on domestic and farm animals. Control of chewing and sucking insects and spider mites on fruit, vegetables, field crops, ornamentals, glasshouse crops, and house plants. Also used as personal insect repellents and for human head lice control. Normally combined with synergists, e.g. piperonyl butoxide, which slows down detoxification by the insects. Formulation types AE; DP; EC; UL; WP; Fogging concentrate; Pressurised liquid CO2. Compatibility Incompatible with alkaline substances. Selected products: 'Evergreen' (MGK); 'ExciteR' (Prentiss); 'Milon' (Frunol); 'Pyrocide' (MGK); 'PY-T-20' (Botanical Resources); 'PY-T-50' (Botanical Resources); mixtures: 'Hash' (+ piperonyl butoxide) (Kemio); 'Pycon' (+ piperonyl butoxide) (Agropharm); 'Pyronyl' (+ piperonyl butoxide) (Prentiss)
OTHER PRODUCTS
pyrethrins (pyrethrum)
'Alfadex' (Novartis A H); 'Pybuthrin 33' (Bayer CropScience); 'Pyganic' (MGK); 'Pyrkem' (Kemio) mixtures: 'Asgrow Thirethrin' (+ endosulfan+ piperonyl butoxide) (Agriliance); 'Hash J25' (+ piperonyl butoxide) (aerosol) (Kemio); 'Parexan' (+ S421+ piperonyl butoxide) (Bayer CropScience); 'Piretrin' (+ piperonyl butoxide) (Chemia); 'Pybuthrin' (+ bioallethrin+ bioresmethrin) (Bayer CropScience); 'Pynosect 30 Water Miscible' (+ resmethrin) (Mitchell Cotts); 'Pyrellin' (+ rotenone) (Wright Webb); 'Pyrenone' (+ piperonyl butoxide) (Bayer CropScience) Discontinued products: 'CheckOut' * (Suterra) mixtures: 'Pydon' * (+ S421) (Protex SA); 'Pynosect 30 Fogging Solution' * (+ resmethrin) (Mitchell Cotts)
ANALYSIS
Products and extracts of flowers analysed by glc with FID (CIPAC Handbook, 1985, 1C, 2209; ibid., 1998, H, 239; AOAC Methods, 17th Ed., 982.02*; D. B. McClellan, Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 461; J. Sherma, ibid., 1976, 8, 225); or by a titrimetric method (AOAC Methods, 17th Ed., 936.05). Residues determined by colorimetry or by glc (J. Sherma, loc. cit.; R. Mestres et al., Ann. Falsif. Expert. Chim., 1979, 72, 577; W. Specht & M. Tillkes, Fresenius' Z. Anal. Chem., 1980, 301, 300).
MAMMALIAN TOXICOLOGY
pyrethrins (pyrethrum)
Reviews FAO/WHO 86, 88 (see part 2 of the Bibliography). Mammalian toxicity has been reviewed (G. P. Schoenig, "Environmental Fate of Pyrethroids" in Pyrethrum Flowers, pp 249-257). Oral Acute oral LD50 for male rats 2370, female rats 1030, mice 273-796 mg/kg. Skin and eye Acute percutaneous LD50 for rats >1500, rabbits 5000 mg/kg. Slightly irritating to skin and eyes. Constituents of the flowers may cause dermatitis to sensitised individuals, but are removed during the preparation of refined extracts. Inhalation LC50 (4 h) for rats 3.4 mg/l. NOEL (2 y) for rats 100 ppm. ADI (JMPR) 0.04 mg/kg b.w. [1999]. Other There is no evidence that synergists increase toxicity of the pyrethrins to mammals. Toxicity class WHO (a.i.) II; EPA (formulation) III EC classification Xn; R20/21/22| N; R50, R53 (for pyrethrins including cinerins)
pyrethrins (chrysanthemates)
EC classification Xn; R20/21/22| N; R50, R53 (pyrethrin I); Xn; R22| N; R50, R53 (cinerin I)
pyrethrins (pyrethrates)
EC classification Xn; R20/21/22| N; R50, R53 (pyrethrin II); Xn; R22| N; R50, R53 (cinerin II)
ECOTOXICOLOGY
pyrethrins (pyrethrum)
Birds Acute oral LD50 for mallard ducks >10 000 mg/kg. Fish Highly toxic to fish. LC50 (96 h) (static tests) for coho salmon 39, channel catfish 114 mg/l. LC50 for bluegill sunfish 10, rainbow trout 5.2 mg/l. Daphnia LC50 12 mg/l. Bees Toxic to bees, but exhibits a repellent effect. LD50 (oral) 22 ng/bee; (contact) 130-290 ng/bee.
ENVIRONMENTAL FATE
Environmental fate has been reviewed (D. G. Crosby, "Environmental Fate of Pyrethroids" in Pyrethrum Flowers, pp 196-213). Animals In mammals, rapidly degraded by oxidation; for reviews, see J. E. Casida & G. B. Quistad, "Metabolism and Synergism of Pyrethrins" in Pyrethrum Flowers, pp 196-213, and Pyrethrum, the Natural Insecticide. Soil/Environment In the environment, degradation, promoted by sunlight and u.v. light, begins at the alcohol group and involves the formation of numerous unknown cleavage products.