64924-67-0氢溴酸常山酮Halofuginone hydrobromide
Halofuginone hydrobromide
64924-67-0
64924-67-0
询价
1KG
起订
江西 更新日期:2026-02-10
产品详情:
- 中文名称:
- 氢溴酸常山酮
- 英文名称:
- Halofuginone hydrobromide
- CAS号:
- 64924-67-0
- 品牌:
- Revere
- 产地:
- 江西
- 保存条件:
- 室内常温密封储存
- 纯度规格:
- ≥98%
- 产品类别:
- 精细化学系列
- CAS编号:
- 64924-67-0
- 别名:
- 氢溴酸常山酮 氢溴酸卤夫酮 卤夫酮氢溴酸盐 氢溴酸溴氯哌喹酮 氢溴酸常山酮|哈洛夫酮 氢溴酸常山酮溶液,100PPM
- 分子式:
- C16H17BrClN3O3.HBr
- 含量纯度:
- ≥98%
- 产地/厂商:
- 江西瑞威尔生物
- 英文名称:
- Halofuginone hydrobromide
- 性状:
- 类白色粉沫/结晶
- 储藏:
- 室内常温密封储存
- 单杂:
- ≤0.1
- 质量标准:
- 企业内控
公司简介
瑞威尔依托峡江医药大健康产业基础,初步形成了生产加工中成药、原料药、医药中间体、保健药、兽药、医疗器械等产品的产业发展格局,大力推进医药化工产业基地建设。
瑞威尔专业从事生物医学技术服务、技术咨询及产品开发的生物技术服务企业。
公司致力于以生物科技、医药科技的技术开发、技术咨询;以及医药中间体、化工产品及化工原料(不含危化品)的研发、销售;其中包括化妆品原料、食品添加剂、饲料添加剂、兽药、农药、香精香料、医药原料、化工原料等产品技术为核心的生产、研发;还包括货物的进出口、技术的进出口。
| 成立日期 | (6年) |
| 注册资本 | 200万 |
| 员工人数 | 1-10人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 贸易,工厂,试剂,定制,服务 |
| 主营行业 | 化学农药原药,中间体,化学试剂,医药原料,日用化工 |
氢溴酸常山酮相关厂家报价 更多
-

- 常山酮氢溴酸盐;含量98%,抗球虫药,氢溴酸常山酮,乳酸常山酮,不耐药
- 武汉普世达生物科技有限公司 VIP
- 2026-02-14
- ¥18000
-

- 氢溴酸常山酮 C A S :64924-67-0
- 湖北省圣宝莱生物科技有限公司 VIP
- 2026-02-11
- 询价
-

- 氢溴酸常山酮
- 湖北阡陌生物科技有限公司 VIP
- 2026-02-11
- ¥1500
-

- 氢溴酸常山酮 Halofuginone hydrobromide 64924-67-0
- 成都彼样生物科技有限公司 VIP
- 2026-02-10
- 询价
-
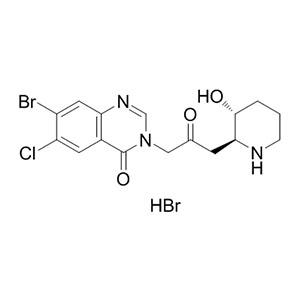
- 氢溴酸卤夫酮 中间体 64924-67-0
- 湖北兴琰新材料科技有限公司 VIP
- 2026-02-07
- 询价
-
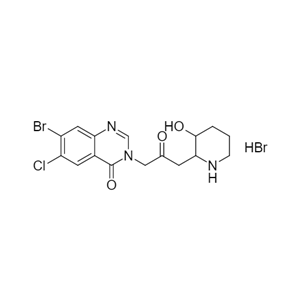
- 常山酮氢溴酸盐
- 奥夫仑医药(四川)有限公司 VIP
- 2026-01-21
- 询价
-
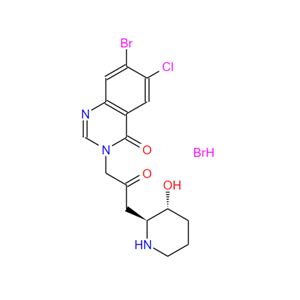
- 64924-67-0;氢溴酸卤夫酮
- 陕西缔都医药化工有限公司 VIP
- 2026-01-21
- 询价
-

- 常山酮溴酸盐|T3524|TargetMol
- TargetMol中国(陶术生物) VIP
- 2025-11-17
- ¥300
-
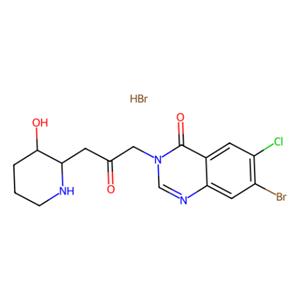
- aladdin 阿拉丁 H288889 溴氯哌喹酮 氢溴酸盐 64924-67-0 ≥98%(HPLC)
- 上海阿拉丁生化科技股份有限公司 VIP
- 2025-05-16
- ¥441.90
-
- 卤夫酮氢溴酸盐
- 湖北隆信化工实业有限公司
- 2023-02-20
- 询价






