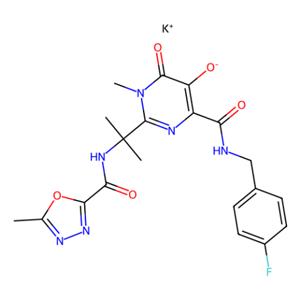
雷特格韦钾盐
Raltegravir potassium
871038-72-1
871038-72-1
询价
10g
起订
100g
起订
500g
起订
湖北 更新日期:2024-07-17
产品详情:
- 中文名称:
- 雷特格韦钾盐
- 英文名称:
- Raltegravir potassium
- CAS号:
- 871038-72-1
- 品牌:
- 湖北凯隆
- 产地:
- 国产
- 保存条件:
- 常温密封干燥储存
- 纯度规格:
- 98HPLC
- 产品类别:
- 原料药
- 性状:
- 白色粉末
- 含量:
- ≥98%
公司简介
湖北凯隆生物科技有限公司坐落于九省通衢之都--湖北武汉,主要致力于原料药、中间体、药用精细化学品的开发与生产,是集研发、生产、销售、咨询服务于一体的科技型企业,公司拥有自己的研发基地,配备了先进的生产设备和高效精密的检测仪器,以高新技术及卓越创新的优质服务赢得了高校机关、科研单位、药厂商、食品相关等行业的广大用户的信任。从而确保我们的产品在同行业中具备超强的竞争力。诚信经营,让客户满意是我们一贯的宗旨;追求品质,为客户提供高质量的产品是我们义不容辞的责任。
| 成立日期 | (2年) |
| 注册资本 | 200万人民币 |
| 员工人数 | 1-10人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 贸易 |
| 主营行业 | 中间体,医药原料 |
雷特格韦钾盐相关厂家报价 更多
-

- 雷特格韦钾盐 871038-72-1
- 武汉维斯尔曼生物工程有限公司 VIP
- 2025-12-31
- 询价
-

- 雷特格韦钾盐
- 武汉裕清嘉衡药业有限公司 VIP
- 2025-12-31
- 询价
-

- 雷特格韦钾盐—871038-72-1
- 湖北魏氏化学试剂股份有限公司 VIP
- 2025-12-30
- 询价
-

- 雷特格韦钾盐
- 湖北海克斯生化有限公司 VIP
- 2025-12-29
- 询价
-

- 871038-72-1;雷特格韦钾盐
- 普善实业(陕西)有限公司 VIP
- 2025-12-26
- 询价
-

- 魏氏化学 雷特格韦钾盐—871038-72-1 科研试剂
- 湖北魏氏化学试剂股份有限公司 VIP
- 2025-12-25
- ¥1359
-

- 雷特格韦钾盐—871038-72-1
- 湖北魏氏化学试剂股份有限公司 VIP
- 2025-12-24
- ¥573
-

- 雷特格韦钾盐|T2239
- 上海陶术生物科技有限公司 VIP
- 2025-10-31
- ¥148
-
- aladdin 阿拉丁 R304804 雷特格韦钾盐 871038-72-1 97%
- 上海阿拉丁生化科技股份有限公司 VIP
- 2025-05-16
- ¥1489.90
-

- Raltegravir (MK-0518)
- 南京百鑫德诺生物科技有限公司
- 2024-09-26
- 询价


