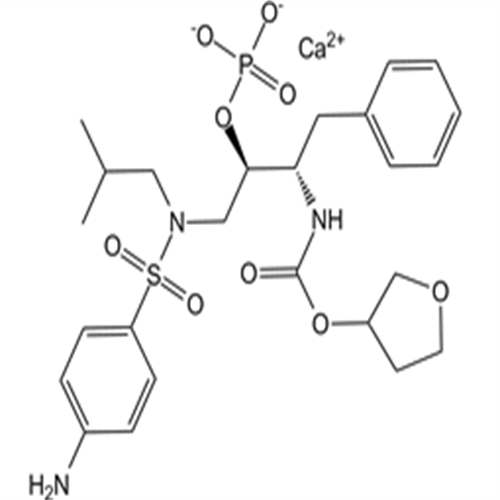
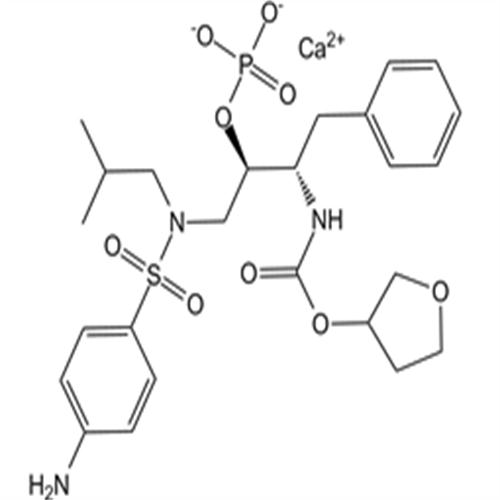
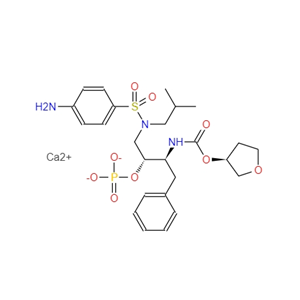
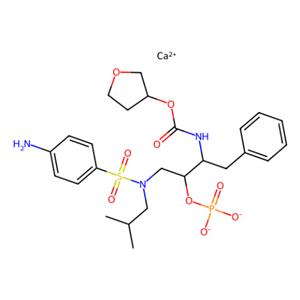
226700-81-8Fosamprenavir Calcium Salt
Fosamprenavir Calcium Salt
226700-81-8
226700-81-8
询价
1盒
起订
上海 更新日期:2025-11-06
产品详情:
- 中文名称:
- Fosamprenavir Calcium Salt
- 英文名称:
- Fosamprenavir Calcium Salt
- CAS号:
- 226700-81-8
- 品牌:
- 一研
- 保存条件:
- Store at -20°C
- 产品类别:
- 抑制剂
- Cas:
- 226700-81-8
公司简介
上海一研生物科技有限公司Shanghai yiyan bio-technology Co. Ltd.主要从事免疫学、分子生物学和常规生化试剂等为一体的科研产品销售企业,公司自成立以来,秉承""全心全意服务于科研工作者""的企业理念,立足生物科技领域,运用生物技术和科研试剂,发展现代生物科技,为各类大中小医院及其它医疗机构、高等院校、科研院所、企事业单位提供优质的产品,服务生物科技领域的科学研究人员。
公司具有对普通货物、冷藏及冷冻仓库的存储、包装及运输能力。
公司将始终坚持信誉立业、以人为本、质量保证、诚信服务的宗旨,不断拼搏,开拓进取,与各界朋友携手共创美好未来。
| 成立日期 | (12年) |
| 注册资本 | 100 |
| 员工人数 | 50-100人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 工厂,试剂 |
| 主营行业 | 生化试剂,抗体,细胞培养,分子生物学,免疫安全 |
Fosamprenavir Calcium Salt相关厂家报价
-
- 福沙那韦钙 226700-81-8 Fosamprenavir calcium
- 湖北威德利化学科技有限公司 VIP
- 2026-03-02
- ¥5500
-

- 226700-81-8;福沙那伟钙
- 普善实业(陕西)有限公司 VIP
- 2026-02-27
- 询价
-

- 福沙那伟钙|T8238|TargetMol
- TargetMol中国(陶术生物) VIP
- 2025-11-17
- ¥543
-
- 福沙那伟钙 226700-81-8
- 宝鸡缔都医药化工有限公司 VIP
- 2025-09-23
- 询价
-
- aladdin 阿拉丁 F334964 福沙普列那韦钙盐 226700-81-8 ≥95%
- 上海阿拉丁生化科技股份有限公司 VIP
- 2025-05-16
- ¥673.90