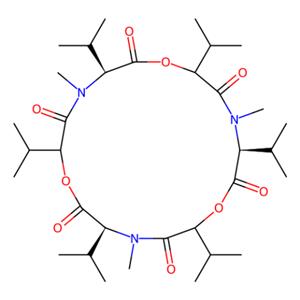
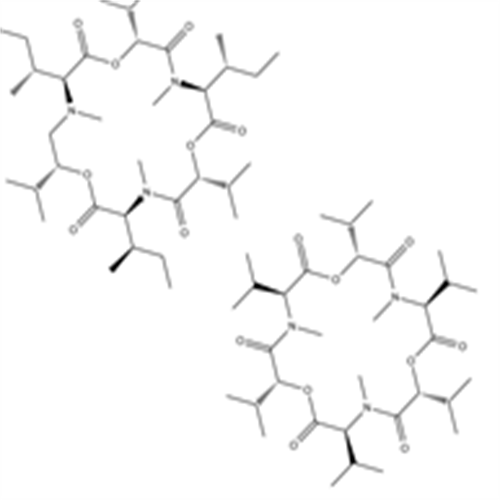
产品属性:
产品名称 | 规格 | CAS号 | 型号 |
Enniatin Complex | 10mg 50mg | 11113-62-5 | EY-Y0164434 |
Cas No.11113-62-5
别名
化学名 (3S,6R,9S,12R,15S,18R)-3,6,9,12,15,18-hexaisopropyl-4,10,16-trimethyl-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexaone compound with (3S,6R,9S,12R,15S,18R)-3,9,15-tri((R)-sec-butyl)-6,12,18-triisopropyl-4,10,16-t
分子量 639.8
溶解度 DMF: soluble,DMSO: soluble,Ethanol: soluble,Methanol: soluble
储存条件 Store at -20°C
General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.
Shipping Condition Evaluation sample solution : ship with blue ice
All other available size: ship with RT , or blue ice upon request
产品描述:
Enniatins, analogues of beauvericin, is a regular cyclic hexadepsipeptide isolated from Fusarium species of fungis, also they have been isolated from other genera, such as Verticillium and Halosarpheia [1]. They have been involved in many biological activities, such as antiinsectan, antifungal, antibiotic and cytotoxic [1].
In vitro: Fusafungine is a mixture of several enniatins with bacteriostatic activity against most micro-organisms responsible for infections and superinfections of the respiratory tract. Fusafungine has been developed for treatment of upper respiratory tract infections by oral and/or nasal inhalation. Fusafungine in low concentrations down-regulated the expression of intercellular adhesion molecule-1 (ICAM-1) by activated macrophages, inhibited the production of the proinflammatory cytokines IL-1, TNFα and IL-6 and inhibited the release of oxygen free radicals by inflammatory macrophages without altering their phagocytic activity. Fusafungine also inhibited T-cell activation and proliferation, and the synthesis of IFN-γ by activated T cells [2]. Exposure 8 h to Enniatins at nanomolar concentrations significantly stimulated cell proliferation. At low micromolar concentrations, enniatins exihibited profound apoptosis-inducing effects against various human cancer cell types. In the fluorescence-activated cell sorting analysis, Enniatins induced cell cycle arrest in the G0/G1 phase. In human cancer cells, elevated ENN concentrations induced profound p53-dependent cytostatic and p53-independent cytotoxic activities [3]. Enniatin easily incorporated into the cell membrane in which it formed cation-selective pores [4].
References:
[1] Sy-Cordero A A, Pearce C J, Oberlies N H. Revisiting the enniatins: a review of their isolation, biosynthesis, structure determination and biological activities[J]. The Journal of antibiotics, 2012, 65(11): 541-549.
[2] German-Fattal M. Fusafungine, an antimicrobial with anti-inflammatory properties in respiratory tract infections[J]. Clinical Drug Investigation, 2001, 21(9): 653-670.
[3] Dornetshuber R, Heffeter P, Kamyar M R, et al. Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells[J]. Chemical research in toxicology, 2007, 20(3): 465-473.
[4] Kamyar M, Rawnduzi P, Studenik C R, et al. Investigation of the electrophysiological properties of enniatins[J]. Archives of biochemistry and biophysics, 2004, 429(2): 215-223.