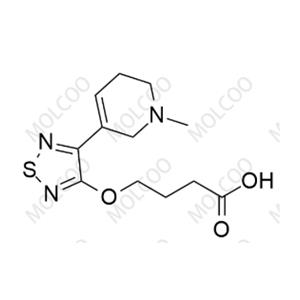
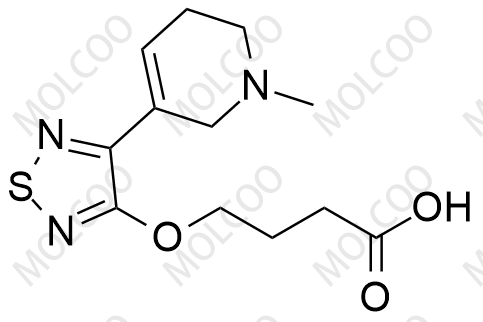
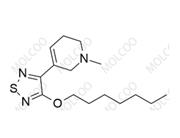
Xanomeline Impurity 159058-46-5
I. Product Overview
Xanomeline Impurity Reference Standard is a high-purity reference material designed for pharmaceutical R&D, drug quality control, and laboratory research. Its precise chemical composition and rigorous quality control ensure reliable analytical results, facilitating compliance with international regulatory standards.
II. Technical Specifications
| Parameter | Details |
|---|
| CAS Number | Varies by impurity type (e.g., Impurity 1: 174656-58-7; Impurity 4: 2414049-88-8) |
| Molecular Formula | Varies by impurity structure (e.g., Impurity 1: C₁₄H₂₃N₃O₂S; Impurity 4: C₁₄H₂₀N₃O₂S) |
| Molecular Weight | Varies by impurity type (e.g., Impurity 1: 297.42 g/mol; Impurity 4: 294.39 g/mol) |
| Purity | ≥95% (COA, HPLC, MS, NMR, and other analytical data provided) |
| Size | 10mg/25mg/50mg/100mg/1g (custom packaging available) |
| Storage Condition | Store at low temperature (-20℃ or as recommended in COA), protect from light and moisture |
III. Compliance & Certification
Standards Compliance: GMP/GLP, ISO, ASTM, and other international quality control standards.
Pharmacopoeial References: Supports procurement of USP, EP, BP, JP, and other pharmacopoeial standards.
Traceability Documents: Each batch includes Certificate of Analysis (COA), MSDS, and structural confirmation spectra (NMR, IR, UV, etc.).
IV. Applications
Drug Submission: For impurity profiling and consistency evaluation to meet regulatory requirements.
Instrument Calibration: As a quantitative benchmark for HPLC, MS, and other analytical instruments.
Scientific Research: Studying drug stability, metabolic pathways, and impurity toxicity.
V. Product Advantages
High Purity Assurance: Rigorous quality control processes ensure experimental reproducibility.
Rapid Response: 20,000+ in-stock items, same-day shipping, and support for urgent orders.
Customization Services: Offering impurity synthesis, purification, and custom development of novel drug impurities.
VI. Packaging & Stability
Packaging: Sealed vials/amber bottles with labels (including CAS number, batch number, expiration date).
Stability: Long-term monitoring data shows a shelf life ≥2 years under low-temperature storage.


 China
China