1/5
Vortioxetine hydrobromide NEW
- Min. Order1KG
- Purity99%
- Cas No960203-27-4
- Supply Ability50000KG/month
- Update time2025-03-21

| Product Name | Vortioxetine hydrobromide |
| CAS No | 960203-27-4 |
| EC-No | 120-321-1 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
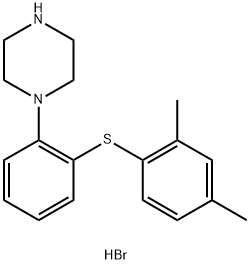
| CAS: | 960203-27-4 |
| MF: | C18H23BrN2S |
| MW: | 379.36 |
| EINECS: | 120-321-1 |
| Product Categories: | 5-HT antagonist;Serotonin transporter inhibitor;Inhibitors;API;960203-27-4 |
| Mol File: | 960203-27-4.mol |
 | |
| Vortioxetine hydrobromide Chemical Properties |
| Melting point | >223°C (dec.) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Pale Beige to Light Beige |
| Vortioxetine hydrobromide Usage And Synthesis |
| Antidepressant | Vortioxetine HBr is a new type of diaryl sulfanyl amine antidepressants developed by Japan Takeda and Denmark Lundbeck for the treatment of depression and anxiety. FDA was admitted to be on American market in September 2013 with trade name Brintellix used for the treatment of severe depression in adults and in October of the same year, the admittance application of Vortioxetine to be listed (MAA) received positive comments from European Medicines Agency (EMA) Human Medicines Products Committee (CHMP). CHMP advised to approve of Brintellix being used for the treatment of adult patients with severe depression (MDD). The EMA European Commission granted Vortioxetine the right to be sold throughout the EU in December 2013 with four dose forms,5 mg, 10 mg, 15 mg and 20 mg. Vortioxetine hydrobromide now has applied to be listed in a number of countries except in China. Vortioxetine HBr is considered to be a new type of multi-model antidepressant. Vitro researches show that it can antagonize 5-HT3,5-HT7and 5-HT1D receptor, activate 5-HT1A receptor, partly activate 5-HT1B receptor and inhibit 5-HT from operation. Vortioxetine is another kind of antidepressant made by Lundbeck aiming at replace Citalopram which is expired patent drug. The pharmacological effect, indication, drug interaction, synthetic method and so on are edited by Chemicalbook's Yao Yao. |
| Vortioxetine | Vortioxetine is an oral immediate release tablet whose main active ingredient is Vortioxetine hydrobromide, which is a kind of antidepressant. Vortioxetine hydrobromide is a slightly yellowish white powder and slightly soluble in water. It is a kind of tablet with different dose forms with each piece containing Vortioxetine hydrobromide 6.335mg,12.71mg,19.065mg or 25.42mg equivalent to 5mg, 10mg, 15mg and 20mg Vortioxetine respectively. The non-active ingredients in Vortioxetine tablet include mnnitol, microcrystalline cellulose, hydroxypropylcellulose, sodium carboxymethyl starch, magnesium stearate and thin film cover made up of hydroxypropylmethylcellulose, titanium dioxide, polyethylene glycol 400, ferric oxide and iron oxide yellow. Clinical evaluation 1. Vortioxetine has a high affinity for 5-HT. Compared with duloxetine, oxetine, it can act on 5-HT3, 5-HT1A, 5-HT7,5-HT1D and 5-HT1B receptors with high selectivity. When used to regulate emotions , the dose is small, with simple medication once a day improving patient’s compliance. The drug has less drug interactions, high selectivity and less side effects. Vortioxetine is a new antidepressant and is considered as the most successful drug in the study of single-phase emotional disorder. The results show that Vortioxetine is a more effective antidepressant compared with placebo and can significantly increase 5-HTT possession rate. It is safer with safety similar to venlafaxine and the incidence of adverse reactions is lower than duloxetine. Another clinical study shows that Vortioxetine can effectively reduce the probability of recurrence after treatment. Animal experiments show that Vortioxetine has a significant effect on behavioral analysis of antidepressant activity. In a number of rat anti-anxiety models, Vortioxetine has higher anti-anxiety activity compared to other known antidepressants and still has effect on the anxiety state on which paroxetine and duloxetine. cannot work. In summary, compared with other antidepressants, Vortioxetine has high efficacy, less adverse reactions and obvious clinical advantages. |
| Biological activity | Vortioxetine (Lu AA21004) HBr is a 5-hydroxytryptamine inhibitor that inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptors and SERT with IC50 being 15nM,33nM, 3.7nM,19nM and1.6nM. |
| Approval | Vortioxetine hydrobromide is an atypical antidepressant made by Lundbeck and Takeda. It was approved by the United States Federal Drug Agency and the Committee for Medicinal Products for Human Use (CHMP) on September 30, 2013 and December 27, 2013 respectively, for the treatment of major depressive disorder in adults. Vortioxetine has also been investigated as a treatment for generalized anxiety disorder. |
| In vitro | Lu-AA21004 inhibits recombine human CYP1A2, CYP2C9, CYP2D6 and CYP3A4 with IC50 being 40μM, 39μM, 9.8μM and 10μM respectively. Lu AA21004 is a h5-HT1B receptor agonist with EC50 being 460nM and in the whole cell detection the intrinsic activity is 22%. In vitro full-cell cAMP assay, Lu AA21004 is a functional antagonist against r5-HT7 receptor with Ki being 200nM. Lu AA21004 is a functional antagonist of r5-HT7 receptor with IC50 being 2μM. |
| In vivo | The clearance on rat liver and oral bioavailability of Lu-AA21004 are 7.1(L/h)/kg and 16% respectively. Lu-AA21004 is injected subcutaneously into the hippocampus of the conscious rats at 2.5mg/kg, 5mg/kg or 10 mg/ kg to increase extracellular 5-HT levels. Lu-AA21004 significantly increases the basal level of 5-HT after being injected into medial prefrontal cortex (mPFC) at the dose of 5mg/kg or 10mg/kg for 3 days. LuAA21004 affects the Bezold-Jarisch reflection of rats, which is dose-dependent and inhibits transient bradycardia with ED50 being 0.11mg /kg. LuAA21004 is injected subcutaneously into the medial prefrontal cortex and ventral hippocampus of the rats at the dose of 2.5-10.0mg/kg to increase the levels of extracellular 5-HT, DA, and NA. LuAA21004 is injected subcutaneously into the ventral hippocampus of rats at dose of 5mg/kg to increase extracellular 5-HT levels (200%). Lu AA21004 (10mg/kg) significantly reduces pain in rats. Lu AA21004 increases the level of ACh to 224% and 204% at dose of 5 and 10mg/kg after 20min. |
| Uses | Vortioxetine Hydrobromide is a multimodal serotonergic agent that inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor and SERT (1,2,3). |
| Definition | ChEBI: Vortioxetine hydrobromide is a hydrobromide obtained by combining vortioxetine with one molar equivalent of hydrobromic acid. Used for treatment of major depressive disorder. It has a role as an antidepressant, an anxiolytic drug, a serotonergic antagonist and a serotonergic agonist. It contains a vortioxetine(1+). |
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-







