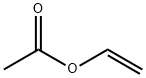
| Product Name | Vinyl acetate |
| CAS No | 108-05-4 |
| EC-No | 203-545-4 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
| CAS: | 108-05-4 |
| MF: | C4H6O2 |
| MW: | 86.09 |
| EINECS: | 203-545-4 |
| Product Categories: | VAM;Vinyl Esters;Building Blocks;C2 to C5;Carbonyl Compounds;Chemical Synthesis;Esters;Materials Science;Monomers;Organic Building Blocks;Polymer Science;108-05-4 |
| Mol File: | 108-05-4.mol |
 | |
| Vinyl acetate Chemical Properties |
| Melting point | -93 °C (lit.) |
| Boiling point | 72-73 °C (lit.) |
| density | 0.934 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 88 mm Hg ( 20 °C) |
| refractive index | n |
| Fp | 20 °F |
| storage temp. | 2-8°C |
| solubility | 20g/l |
| form | Liquid |
| color | Clear colorless to almost colorless |
| Odor | char. odor |
| PH | 7 (20g/l, H2O, 20℃) |
| explosive limit | 2.6-13.4%(V) |
| Water Solubility | 23 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,9992 |
| BRN | 1209327 |
| Henry's Law Constant | 4.81 (calculated, Howard, 1989) |
| Exposure limits | NIOSH REL: 15-min ceiling 4 ppm (15 mg/m3); ACGIH TLV: TWA 10 ppm, STEL 15 ppm (adopted). |
| Stability: | Stable. Highly flammable. Incompatible with acids, bases, oxidizing agents, peroxides, chlorosulfonic acid, ethylene imine, hydrochloric acid, oleum, nitric acid, sulfuric acid, 2-aminoethanol, light. Susceptible to polymerization; commercial product may be stabilized by the addition of hydroquinone. |
| LogP | 0.73 at 20℃ |
| CAS DataBase Reference | 108-05-4(CAS DataBase Reference) |
| IARC | 2B (Vol. Sup 7, 63) 1995 |
| NIST Chemistry Reference | Acetic acid ethenyl ester(108-05-4) |
| EPA Substance Registry System | Vinyl acetate (108-05-4) |
| Vinyl acetate Usage And Synthesis |
| Description | Vinyl acetate monomer (VAM) is a colourless liquid, immiscible or slightly soluble in water. VAM is a flammable liquid. VAM has a sweet, fruity smell (in small quantities), with sharp, irritating odour at higher levels. VAM is an essential chemical building block used in a wide variety of industrial and consumer products. VAM is a key ingredient in emulsion polymers, resins, and intermediates used in paints, adhesives, coatings, textiles, wire and cable polyethylene compounds, laminated safety glass, packaging, automotive plastic fuel tanks, and acrylic fibres. Vinyl acetate is used to produce polyvinyl acetate emulsions and resins. Very small residual levels of vinyl acetate have been found present in products manufactured using VAM, such as moulded plastic items, adhesives, paints, food packaging containers, and hairspray. |
| Chemical Properties | Vinyl acetate is a colorless, flammable liquid with a pungent odor. The odor threshold is 0.12 ppm 0.3 ppm (NY, NJ).it is the precursor to polyvinyl acetate, an important polymer in industry. |
| Physical properties | Colorless, watery liquid with a pleasant, fruity odor. Experimentally determined detection and recognition odor threshold concentrations were 400 μg/m3 (120 ppbv) and 1.4 mg/m3 (400 ppbv), respectively (Hellman and Small, 1974). |
| Uses | Vinyl acetate is primarily used to produce polyvinyl acetate emulsions and polyvinyl alcohol. The principal use of these emulsions has been in adhesives, paints, textiles, and paper products. |
| Uses | In polymerized form for plastic masses, films and lacquers; in plastic film for food packaging. As modifier for food starch. |
| Production Methods | Vinyl acetate is an industrial chemical that is produced in large amounts in the United States. The worldwide production capacity of vinyl acetate monomer (VAM) was estimated at 6,154,000 tonnes/annum in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000) . The average list price for 2008 was $1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7 %), Chang Chun Group (6%) and LyondellBasell (5%). It is a key ingredient in furniture-glue. |
| Preparation | The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst. Ethylene + acetic acid + 1/2 O2 → Vinyl acetate +H2O But by products are also generated: Ethylene + 3 O2 → 2CO2 + 2H2O Vinyl acetate is also prepared by the gas-phase addition of acetic acid to acetylene. |
| Definition | ChEBI: Vinyl acetate is an acetate ester. |
| Reactions | Vinyl acetate undergoes many of the reactions anticipated for an alkene and an ester. Bromine adds to give the dibromide. Hydrogen halides add to give 1-haloethyl acetates, which cannot be generated by other methods because of the non - availability of the corresponding halo-alcohols. Acetic acid adds in the presence of palladium catalysts to give ethylidene diacetate, CH3CH(OAc)2. It undergoes transesterification with a variety of carboxylic acids.The alkene also undergoes Diels - Alder and 2 + 2 cycloadditions. |
| General Description | Vinyl acetate appears as a clear colorless liquid. Flash point 18 °F. Density 7.8 lb / gal. Slightly soluble in water. Vapors are heavier than air. Vapors irritate the eyes and respiratory system. May polymerize if heated or contaminated. If polymerization occurs inside a container, the container may violently rupture. Used to make adhesives, paints, and plastics. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. |
| Reactivity Profile | Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Reacts with hydrogen peroxide to form explosive peracetic acid. Reacts with oxygen to form explosive peroxides. Forms explosive Vinyl acetate ozonide on contact with ozone. Undergoes violent or explosive reactions with 2-aminoethanol, chlorosulfonic acid, ethylenediamine, mineral acids (hydrochloric acid, hydrofluoric acid, nitric acid, sulfuric acid, oleum), and peroxides [Lewis, 3rd ed., 1993, p. 1311]. Polymerization initiated by dibenzoyl peroxide in ethyl acetate accelerated out of control, ignited and exploded [Vervalin, 1973, p. 81]. Polymerization in toluene solution has caused several large industrial explosions [MCA Case History No. 2087]. |
| Health Hazard | Vinyl acetate has been related to reproductive abnormalities. It is a skin and upper respiratory tract irritantand a central nervous system depressant. Exposure caused gradual deterioration of heart muscles. |
| Fire Hazard | When heated to decomposition, Vinyl acetate burns and emits acrid fumes. Highly dangerous when exposed to heat, flames or oxidizers; explosion hazard with strong acids and strong oxidizers. Incompatible with alumina, oxidizing materials, 2-aminoethanol, chlorosulfonic acid; ethyleneimine; 36% hydrochloric acid; 48.7% hydrofluoric acid; 70% nitric acid; oleum; 96% sulfuric acid; ethylene diamine; peroxides and silica gel. Avoid light or any polymerizing initiator. Hazardous polymerization can be initiated by organic and inorganic peroxides; azo compounds; redox systems (including organometallic components); light; and high energy radiation. |
| Flammability and Explosibility | Highly flammable |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-








