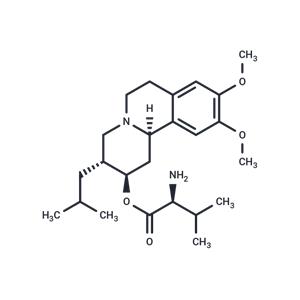
| Name | Valbenazine |
| Description | Valbenazine (NBI-98854), a highly selective vesicular monoamine transporter 2(VMAT2) inhibitor, is approved for the treatment of tardive dyskinesia. |
| Animal Research | The pooled long-term exposure (LTE) population included valbenazine-treated subjects from 3 studies: KINECT; KINECT 3; KINECT4. Safety assessments included adverse events (AEs), laboratory tests, vital signs, electrocardiograms (ECGs), and extrapyramidal symptom (EPS) scales. Psychiatric stability was monitored using the Positive and Negative Syndrome Scale (PANSS) and Calgary Depression Scale for Schizophrenia (CDSS) (SCHZ subgroup), as well as the Montgomery-?sberg Depression Rating Scale (MADRS) and Young Mania Rating Scale (YMRS) (mood subgroup). All data were analyzed descriptively[1]. |
| In vivo | Valbenazine appeared to be well tolerated in adults with TD who received up to 48 weeks of treatment. In addition to long-term efficacy results, Suggest that valbenazine may be appropriate for the long-term management of TD regardless of underlying psychiatric diagnosis (SCHZ disorder or mood disorder)[1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (119.45 mM)
|
| Keywords | disease | VMAT2 | NBI 98854 | Monoamine Transporter | monoamine | transporter | inhibit | Huntington | Inhibitor | vesicular | Valbenazine | NBI98854 |
| Inhibitors Related | Diclofensine | Nisoxetine hydrochloride | Fostamatinib | Tetrabenazine | Rose Bengal sodium | Tetrabenazine Racemate | NBI-98782 | Tetrabenazine Metabolite | (+)-Tetrabenazine | Reserpine | (+)-Kavain | Xanthoangelol |
| Related Compound Libraries | Bioactive Compound Library | Membrane Protein-targeted Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | Ion Channel Targeted Library | Anti-Cancer Drug Library |

 United States
United States