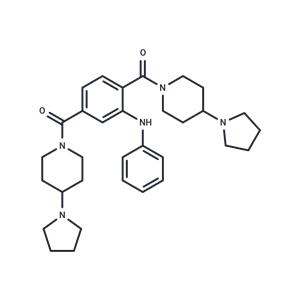
| Name | UNC1215 |
| Description | UNC1215, an effective and specific MBT (malignant brain tumor) antagonist, binds L3MBTL3 (IC50/Kd: 40/120 nM). The selectivity of UNC1215 for L3MBTL3 is 50-fold higher versus other members of the human MBT family. |
| Cell Research | CellTiter-Glo luminescent cell viability assay(Only for Reference) |
| Kinase Assay | AlphaScreen assay: Compound plates (1 μL at 10 or 30 mM highest concentration) are diluted in 1×assay buffer (20 mM Tris pH 8.0, 25 mM NaCl, 2 mM DTT and 0.05% Tween-20) over 2 steps using a Multimek robotic pipettor and 1 μL is spotted into the wells of 384-well assay Proxiplates. To these plates 9 μL of protein- peptide mix in 1× assay buffer is added by Multidrop and incubated for 30 min at room temperature. At this point 2 μL of streptavidin-conjugate donor and nickel-chelate acceptor beads (45 μg/mL in 1× assay buffer) are added, the plates are allowed to incubate for an additional 30 min in the dark at room temperature. After incubation the plates are read on EnVision mulilabel reader equipped with HTS alpha screen laser. The screens reported are performed up to 10 or 30 μM, and therefore it should be noted that those compounds referred to as inactive are indeed inactive only within the concentration range tested. PHF23 and JARID1A are GST tagged and consequently for these assays GST-acceptor beads are used. It should be noted that any positive binding curves for L3MBTL4 that are generated yielded curves with very shallow slopes, suggesting a nonspecific interaction. The data for the IC50 values is calculated from replicate runs in that the datapoints for each compound concentration are averaged and plotted using 4-parameters curve fitting. |
| In vivo | UNC1215 binds to L3MBTL3 and competitively displaces peptides containing mono- or dimethyl-lysine.UNC1215 is approximately 75-fold more selective for L3MBTL3 than L3MBTL1.UNC1215 is non-cytotoxic and binds directly to L3MBTL3 through the Kme-binding pocket of the MBT domain.UNC1215 enables the cellular mobility of GFP-L3MBTL3 fusion proteins and the cellular mobility of the fusion protein. UNC1215 increased cell mobility and point mutations of the GFP-L3MBTL3 fusion protein, interfered with the Kme-binding function of the GFP-L3MBTL3 phenotypic mimic, and affected the localization of UNC1215.UNC1215 (30 μM) did not affect the tandem Tudor domain of UHRF1, the chromatin domain of CBX7, and the PHD domain of JARID1A. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 93 mg/mL (175.6 mM)
H2O : < 1 mg/mL (insoluble or slightly soluble)
Ethanol : 93 mg/mL (175.6 mM)
|
| Keywords | inhibit | Inhibitor | Histone Methyltransferase | Cerebrum | UNC-1215 | probe | Malignant | MBT | methyllysine | Apoptosis | UNC 1215 | UNC1215 |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Acetylcysteine | Kaempferol | Myricetin | Sodium 4-phenylbutyrate | L-Ascorbic acid | Dextran sulfate sodium salt (MW 4500-5500) | Metronidazole | Sorafenib | Tributyrin | Curcumin |
| Related Compound Libraries | Histone Modification Compound Library | Bioactive Compound Library | Epigenetics Compound Library | Chromatin Modification Compound Library | Inhibitor Library | NO PAINS Compound Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Compound Library | Anti-Cancer Active Compound Library |

 United States
United States