WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Number: T107001
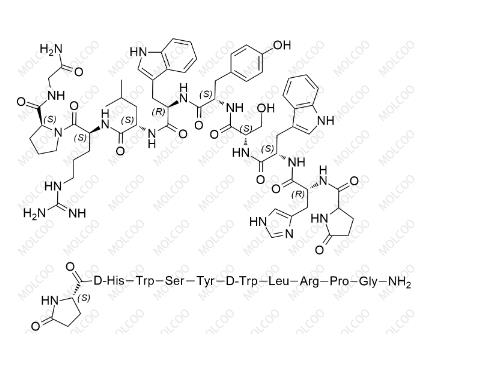
English Name: Triptorelin Impurity 1
English Alias: Pyr-DHis-Trp-Ser-Tyr-DTrp-Leu-Arg-Pro-Gly-NH2; (2S)-1-((3R,6S,9S,12S,15R,18S,21S)-3-((1H-imidazol-4-yl)methyl)-6,15-bis((1H-indol-3-yl)methyl)-21-(3-guanidinopropyl)-12-(4-hydroxybenzyl)-9-(hydroxymethyl)-18-isobutyl-1,4,7,10,13,16,19-heptaoxo-1-(5-oxopyrrolidin-2-yl)-2,5,8,11,14,17,20-heptaazadocosan-22-oyl)-N-(2-amino-2-oxoethyl)pyrrolidine-2-carboxamide
CAS Number: 321709-34-6
Molecular Formula: C64H82N18O13
Molecular Weight: 1311.45
Triptorelin is a gonadotropin-releasing hormone (GnRH) analog, which is clinically used in the treatment of various diseases, such as prostate cancer, breast cancer, endometriosis, and precocious puberty. It binds to the GnRH receptor to regulate the secretion of pituitary gonadotropins, thereby affecting the levels of sex hormones. Triptorelin Impurity 1 is an impurity that may be produced during the production process of triptorelin. The generation of impurities may be related to various factors, such as the quality of raw materials, the complexity of the synthesis process, and the control of reaction conditions. The presence of impurities may have potential impacts on the quality, stability, safety, and effectiveness of triptorelin. Therefore, studying and controlling it is the key to ensuring the quality of triptorelin drugs.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!




 China
China

