| CAS: | 617-86-7 |
| MF: | C6H16Si |
| MW: | 116.28 |
| EINECS: | 210-535-3 |
| Product Categories: | 1;Silanes;Other Reagents;Reduction;Synthetic Reagents;organosilicon compound;Si (Classes of Silicon Compounds);Si-H Compounds;Silicon Compounds (for Synthesis);Synthetic Organic Chemistry;Alkyl Silanes;Hydrogensilanes Hydrogensiloxanes;Reducing Agents;Chemistry;Organics;bc0001 |
| Mol File: | 617-86-7.mol |
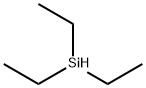 |
|
| Triethylsilane Chemical Properties |
| Melting point | -157°C |
| Boiling point | 107-108 °C (lit.) |
| density | 0.728 g/mL at 25 °C (lit.) |
| vapor pressure | >1 hPa (20 °C) |
| refractive index | n20/D 1.412(lit.) |
| Fp | 25 °F |
| storage temp. | Store below +30°C. |
| solubility | insol H2O; sol hydrocarbons, halocarbons, ethers. |
| form | liquid |
| color | colorless |
| Specific Gravity | 0.728 |
| Water Solubility | Miscible with water. |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 3: reacts with aqueous base |
| BRN | 1098278 |
| Stability: | Stable, but moisture sensitive. Highly flammable. Generates highly flammable gas (hydrogen) in contact with water, acids and bases. |
| LogP | 3.6 at 20℃ |
| CAS DataBase Reference | 617-86-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, triethyl-(617-86-7) |
| EPA Substance Registry System | Triethylsilane (617-86-7) |
| Triethylsilane Usage And Synthesis |
| Description | Triethylsilane is a useful versatile reductant since it has a active hydride. It can be used for mediated the palladium-catalyzed dehalogenation reaction of alkyl or aryl halides, the reduction of primary, secondary, and tertiary chlorides, bromides, and iodides catalyzed by iridium, as well as efficient reduction of multiple bonds, azides, imines, and nitro groups, as well as benzyl group and allyl group deprotection. It is also specifically for the hydrosilation of olefins to give alkyl silanes. It is also the catalyst for synthesis of a spiro-oxindole blocker of Nav1.7 for the treatment of pain, redox initiated cationic polymerization and Beckmann rearrangement of cyclododecanone oxime as well as regioselective reductive coupling of enones and allenes. |
| Chemical Properties | Clear liquid |
| Uses | Triethylsilane is a trialkylsilicon hydride used in the synthesis of alkylsilanes via hydrosilation of olefins. It acts as a reducing agent in the reduction of 2-chromanols, since it has an active hydride. It acts as a catalyst for redox initiated cationic polymerization, regioselective reductive coupling of enones and allenes, and Beckmann rearrangement of cyclododecanone oxime. It is associated with trifluoroacetic acid and involved in the selective reduction of alkenes. |
| Uses | Triethylsilane serves as an exemplar for organo�silicon hydride behavior as a mild reducing agent. It is frequently chosen as a synthetic reagent because of its availability, convenient physical properties, and economy relative to other organosilicon hydrides which might otherwise be suitable for effecting specific chemical transformations.
This reagent is generally used in these reactions: Hydrosilylations, Silane Alcoholysis, Formation of Singlet Oxygen, Reduction of Acyl Derivatives to Aldehydes, Radical Chain Reductions, Ionic Hydrogenations and Reductive Substitutions(The polar nature of the Si–H bond enables triethylsilane to act as a hydride donor to electron-deficient centers.), Reductive Etherifications and Acetal Reductions, Ether Cleavages, Reductive Couplings and Cyclizations, Aromatic Silylations, Generation of Other Triethylsilyl Reagents, etc.
Related Reagents: Phenylsilane–cesium fluoride; tri-n-butylstannane; tricarbonylchloroiridium–diethyl(methyl) silane–carbon monoxide; triethylsilane–trifluoroacetic acid. |
| Application | Triethylsilane is used as a reducing agent inmany organic synthetic reactions.
Used to reduce metal salts. Enhances deprotection of t-butoxycarbonyl-protected amines and tert-butylesters.
Used in the reductive amidation of oxazolidinones with amino acids to provide dipeptides.
Converts aldehydes to symmetrical and unsymmetrical ethers.
Used in the "in-situ" preparation of diborane and haloboranes. |
| Definition | ChEBI: Triethylsilane is an organosilicon compound. It has a role as a reducing agent. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
