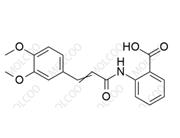
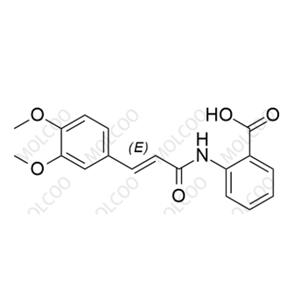
Tranilast Impurity Reference Standard
I. Product Overview
The Tranilast Impurity Reference Standard is a chemical reference standard used for content determination and quality control. This product is crucial for ensuring the purity, quality, and consistency of Tranilast (Cas No. 53902-12-8) medications.
II. Product Information
Chinese Name: Tranilast Impurity Reference Standard
English Name: Tranilast Impurity Reference Standard
CAS Number: Depends on the specific impurity type (Tranilast CAS No. is 53902-12-8)
Molecular Formula and Molecular Weight: Depends on the specific impurity type (Tranilast's molecular formula is C18H17NO5, and its molecular weight is 327.34)
Form: Powder
Storage Conditions: Typically stored at 2-8°C to maintain its stability and activity.
III. Uses and Applications
The Tranilast Impurity Reference Standard is primarily used in drug research and development, production, quality control, and regulatory inspections. It helps identify, quantify, and control impurities in Tranilast medications, ensuring their safety and effectiveness.
IV. Quality Assurance
Our Tranilast Impurity Reference Standard undergoes rigorous quality control and testing to ensure it meets international and domestic standards and regulations. We also provide detailed product specifications and test reports to help customers better understand and use the product.




 China
China