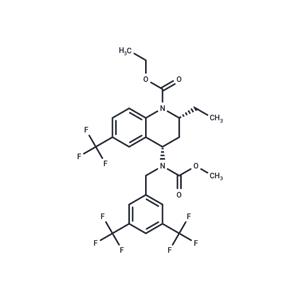
| Name | Torcetrapib |
| Description | Torcetrapib (CP-529414) is a cholesteryl ester transfer protein (CETP) inhibitor designed to reduce the heterotypic transfer of cholesteryl ester from HDL to LDL and/or VLDL. It failed in phase III trials due to an excess of deaths. |
| In vitro | In healthy young adults, daily doses of Torcetrapib at 10 mg, 30 mg, 60 mg, 120 mg, and twice daily at 120 mg increased plasma high-density lipoprotein cholesterol (HDL-C) levels by 16%, 28%, 62%, 73%, and 91%, respectively, without significant changes in total plasma cholesterol (TPC). In rabbits fed an atherogenic diet, Torcetrapib (90 mg/kg/day) more than tripled the plasma HDL-C levels and increased apoA-I levels by 2.5 times. For healthy individuals and patients with moderate hypercholesterolemia, 60 mg and 120 mg daily doses of Torcetrapib raised HDL cholesterol levels by 50% and 60%, respectively. The 60 mg daily dosage enhanced HDL-mediated net cholesterol efflux primarily by increasing HDL concentration, whereas the 120 mg daily dosage did so both by raising HDL concentration and by enhancing efflux at matched HDL concentrations. In the healthy young adult group, taking less than 100 mg of Torcetrapib altered the plasma distribution of cholesteryl ester transfer protein (CETP) 2 hours post-administration, as evidenced by an apparent shift in CETP to larger molecular forms. For patients at high risk of cardiovascular disease, 12 hours post-treatment with Torcetrapib led to a 72.1% increase in HDL-C, a 24.9% decrease in low-density lipoprotein cholesterol (LDL-C), a rise in systolic blood pressure of 5.4 mm Hg, and alterations in serum potassium, sodium, bicarbonate, and aldosterone concentrations. |
| In vivo | Torcetrapib (1 μM) significantly enhances the expression of the steroidogenic genes CYP11B2 and CYP11B1 in the H295R cell line. Treatment with Torcetrapib for 24 or 48 hours increases aldosterone release from H295R cells in a dose-dependent manner, with an EC50 of approximately 80 nM. This effect is mediated by calcium channels, as calcium channel blockers completely inhibit the corticosteroid release and calcium increase induced by Torcetrapib. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 30 mg/mL (50 mM)
|
| Keywords | high-density | cholesterol | Torcetrapib | inhibit | plasma | CP 529414 | low-density | cholesteryl | Inhibitor | lipoprotein LDL | HDL | transfer | CETP | triglycerides | protein | ester | Cholesteryl ester transfer protein | VLDL | CP529414 | lipoprotein |
| Inhibitors Related | Anacetrapib | Granotapide | BMS-212122 | KD-026 | NAMI-A | Lomitapide | Dirlotapide | Obicetrapib | Dalcetrapib | Lomitapide Mesylate |
| Related Compound Libraries | Bioactive Compound Library | ReFRAME Related Library | Anti-Obesity Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Lipid Metabolism Compound Library | Clinical Compound Library | Bioactive Compounds Library Max | Fluorochemical Library | Bioactive Lipid Compound Library |

 United States
United States