Product Code: T036006B
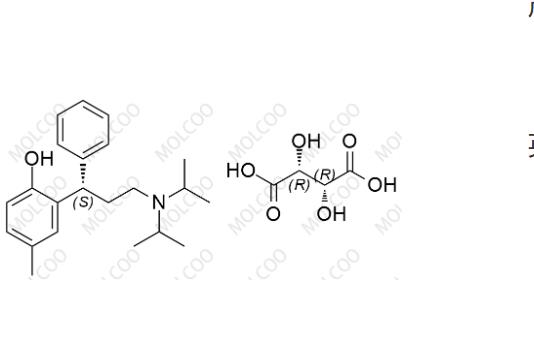
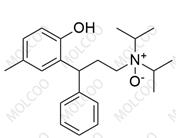
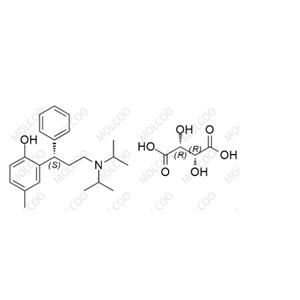
English Name: Tolterodine EP Impurity F(Tartrate)
English Alias: (S)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-methylphenol (2R,3R)-2,3-dihydroxysuccinate
CAS No.:873551-03-2
Molecular Formula: C₂₂H₃₁NO·C₄H₆O₆ (tartrate form)
Molecular Weight: 475.58 (total, 325.49+150.09)
High-Purity Reference Standard: As a reference standard for Tolterodine EP Impurity F (tartrate), its structure is confirmed by NMR, MS, and elemental analysis with ≥99.0% purity (HPLC), stable at 2-8°C in the dark for 24 months.
Regulatory Compliance: Meets EP requirements for impurity reference standards, ensuring high batch-to-batch consistency for impurity control in drug R&D and production.
Quality Control: Used for HPLC detection of Impurity F in Tolterodine API and formulations, controlling content ≤0.1% according to EP standards.
Analytical Method Validation: Serves as a reference standard to develop and validate detection methods for Impurity F in Tolterodine, evaluating method specificity and sensitivity.
Stability Studies: Tracks the formation of Impurity F in Tolterodine stability tests, providing data for storage conditions and shelf life.
Tolterodine is an anticholinergic drug used for treating overactive bladder. Impurity F may arise from residual raw materials, reaction by-products, or degradation during synthesis or formulation. As a specific impurity listed in EP, control of Impurity F is a key part of Tolterodine's quality system, as its presence may affect drug safety and efficacy.
Detection Technology: HPLC-UV with a C18 column (4.6×250mm, 5μm), mobile phase methanol-phosphate buffer (60:40, v/v), detection at 220nm, with LOQ of 0.05%.
Formation Mechanism: Impurity F may originate from alkylation side reactions of phenolic hydroxyl groups in Tolterodine synthesis or amination reactions due to residual isopropylamine; optimizing reaction temperature and purification processes reduces its formation.
Regulatory Requirements: EP specifies an impurity limit of ≤0.1% for Impurity F in Tolterodine, driving the use of this reference standard in pharmaceutical quality control.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China