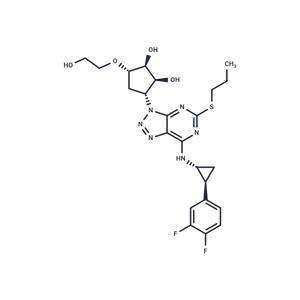
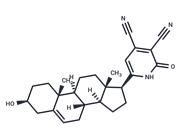
| Name | Ticagrelor |
| Description | Ticagrelor (AR-C 126532XX), produced by AstraZeneca, is an inhibitor of platelet aggregation. Unlike clopidogrel, ticagrelor is not a prodrug required metabolic activation. The drug was approved for use in the European Union by the European Commission on December 3, 2010, and by the US FDA on July 20, 2011. Its trade names are Brilinta (US), Brilique(EU) and Possia(EU). |
| In vitro | Ticagrelor has a half-life (t1/2) of approximately 7-8.5 hours and exhibits a dose-related inhibition of platelet aggregation; a 100-400 mg dose achieves complete inhibition within 2 hours. It is well-tolerated, with no serious adverse events or significant changes in laboratory values observed. Ticagrelor is rapidly absorbed, reaching peak levels in 1.3-2 hours. Within the studied dosage range, both the peak concentration of Ticagrelor and the area under the curve (from time zero to infinity) increase in a dose-proportional manner, indicating linear pharmacokinetics. |
| In vivo | In binding studies on CHO-K1 cells transfected with rh-P2Y12 receptors, Ticagrelor exhibited efficient and reversible binding, with a kon (association rate constant) of 0.00011/(nM·s), a Kd (equilibrium dissociation constant) of 10.5 nM, and a koff (dissociation rate constant) of 0.00087/s. The half-lives for association and dissociation were 4 and 14 minutes, respectively, suggesting that the concentration of the drug that binds to platelets determines the extent of platelet inhibition. Ticagrelor is an active drug that does not require metabolic activation. It does not directly compete with ADP at the ADP binding site but rather occupies a nearby site, causing a change in the conformation of the binding site that leads to a reversible conformational change in the receptor. The binding of Ticagrelor to the receptor is reversible with quick onset/off rates. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (95.68 mM)
5% DMSO+40 % PEG300+5 % Tween 80+50 % Saline : 5 mg/mL (9.57 mM), Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately.
|
| Keywords | Ticagrelor | AZD-6140 | P2Y Receptor | AZD 6140 | Inhibitor | inhibit |
| Inhibitors Related | Tebuconazole | 1-Ethynylnaphthalene | Apigenin | Gemfibrozil | Fenofibrate | 1-Aminobenzotriazole | Uridine-5'-diphosphate disodium salt | Naringin | Naringenin | Tauroursodeoxycholate |
| Related Compound Libraries | Bioactive Compound Library | Membrane Protein-targeted Compound Library | EMA Approved Drug Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | GPCR Compound Library | Anti-Cancer Drug Library |

 United States
United States