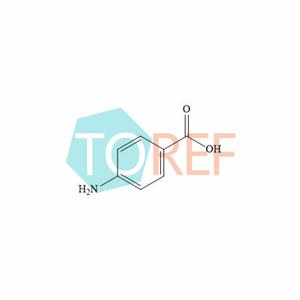
Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
CAS number: 150-13-0
Product Code: REF-T55002
Molecular formula: C7H7NO2
Molecular weight: 137.14
Purity: 97.95%
Product nature: Customized for customers
Appearance characteristics: N/A
Storage conditions: 2-8 ℃
Chemical name: 4-aminobenzoic acid
Hot Tags:Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G), China, suppliers, manufacturers, factory, customized, price, pricelist, in stock。
Synthesis of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G) Reference Standards,
Complex Impurity Of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Chemical Standards of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Characterization Of Unknown Impurities of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Structure Profiling of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Identification of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Isolation & Purification of Impurity of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Difficult of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Instability of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Low-assay of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
Complex Impurity Of Chemical Standards of Tetracaine(Oxybuprocaine)EP Impurity A(Benzocaine EP impurity G)
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.




 China
China